We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1165
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
==How These Structures Lead to Function== | ==How These Structures Lead to Function== | ||
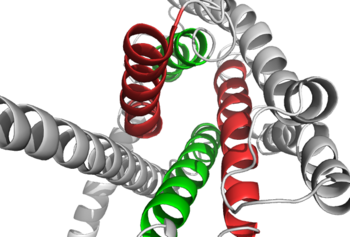
| - | [[Image:Closed and open conformation.png|(|):|250 px|left|thumb|'''Figure | + | [[Image:Closed and open conformation.png|(|):|250 px|left|thumb|'''Figure 2: Open conformation in contrast to the closed conformation.''' The movement of the single helix over the top of the transmembrane domain is the most distinguishable characteristic between closed and open conformation. The <scene name='72/721535/Opening_orientation/2'>stalk</scene> is not accessible to glucagon in the closed conformation.<ref name="Lastt">PMID: 26227798</ref>]] |
| - | Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels. <ref name="Tips">PMID: 23863937</ref> The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide [https://en.wikipedia.org/wiki/Receptor_(biochemistry) receptors]. <ref name="Ligands">PMID: 21542831</ref> The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed [https://en.wikipedia.org/wiki/Conformation conformation] of the GCGR (Figure | + | Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels. <ref name="Tips">PMID: 23863937</ref> The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide [https://en.wikipedia.org/wiki/Receptor_(biochemistry) receptors]. <ref name="Ligands">PMID: 21542831</ref> The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed [https://en.wikipedia.org/wiki/Conformation conformation] of the GCGR (Figure 2). The open conformation is when glucagon can bind to GCGR; in the closed conformation binding does not occur.<ref name="Ligands">PMID: 21542831</ref> |
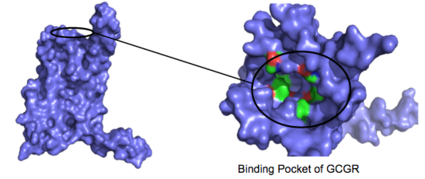
| - | [[Image:Screen_Shot_2016-03-22_at_5.28.03_PM.png|(|):|425 px|center|thumb|'''Figure | + | [[Image:Screen_Shot_2016-03-22_at_5.28.03_PM.png|(|):|425 px|center|thumb|'''Figure 3: Binding Pocket Residues:''' Residues with side chains of carbon(utilizing the [https://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic effect]) are shown in green and side chains containing oxygen ([https://en.wikipedia.org/wiki/Hydrophile hydrophilic]) are shown in red. The properties of hydrophobicity and hydrophilicity of the residues create the [https://en.wikipedia.org/wiki/Ligand_%28biochemistry%29#Receptor.2Fligand_binding_affinity binding affinity] of glucagon.<ref name="Ligands">PMID: 21542831</ref> This image depicts the open conformation of GCGR.]] |
| - | + | The [https://en.wikipedia.org/wiki/Residue_(chemistry) residues] in the binding pocket that are in direct contact with the glucagon molecule are [https://en.wikipedia.org/wiki/Chemical_polarity polar] (utilizing the attraction of opposite charges or (https://en.wikipedia.org/wiki/Dipole dipoles] for glucagon binding) or are hydrophobic (utilizing the hydrophobic effect). The [https://en.wikipedia.org/wiki/Active_site binding site] location of the hormone peptide ligand has been identified, and the N-terminus of glucagon is known to bind partly with the ECD while the rest of glucagon binds deep into the <scene name='72/721535/Binding_pocket_orange/1'>binding pocket</scene> (Figure 3). The [https://en.wikipedia.org/wiki/Amino_acid amino acids] at the N-terminus of the class B 7TM have the ability to form [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds] and [https://en.wikipedia.org/wiki/Ionic_bonding ionic interactions], which can be seen in the [https://en.wikipedia.org/wiki/Peptide_sequence amino acid sequence] of glucagon (Figure 4). <ref name="Sequence">PMID: 11946536</ref> | |
| - | [[Image:Aminoacidsequenceglucagon.png|(|):|425 px|center|thumb|'''Figure | + | [[Image:Aminoacidsequenceglucagon.png|(|):|425 px|center|thumb|'''Figure 4: Amino Acid Sequence of Glucagon''']] |
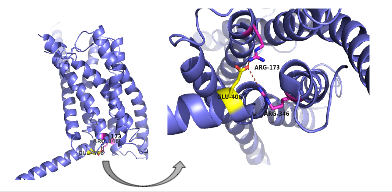
| - | There are specific amino acid interactions that maximize affinity. This includes the alpha helical structure of the <scene name='72/721535/Opening_orientation/2'>stalk</scene>. The alpha helical structure of the stalk interacts directly with glucagon; when the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases. Furthermore, there are certain interactions that hold the helices of the 7TM in the conformation that maximizes [http://www.chemicool.com/definition/affinity.html affinity]. <ref name="Ligands">PMID: 21542831</ref> The high affinity conformation of GCGR is the open conformation when glucagon can bind. Without these specific interactions between the residues, open conformation is not stabilized and GCGR remains in the closed conformation where glucagon cannot bind. <ref name="Tips">PMID: 23863937</ref> The [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin/1'> Cys 294 and Cys 224</scene> serves to hold the helices in the proper orientation for binding and | + | There are specific amino acid interactions that maximize affinity. This includes the alpha helical structure of the <scene name='72/721535/Opening_orientation/2'>stalk</scene>. The alpha helical structure of the stalk interacts directly with glucagon; when the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases. Furthermore, there are certain interactions that hold the helices of the 7TM in the conformation that maximizes [http://www.chemicool.com/definition/affinity.html affinity]. <ref name="Ligands">PMID: 21542831</ref> The high affinity conformation of GCGR is the open conformation, when glucagon can bind. Without these specific interactions between the residues, the open conformation is not stabilized and GCGR remains in the closed conformation, where glucagon cannot bind. <ref name="Tips">PMID: 23863937</ref> The [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin/1'> Cys 294 and Cys 224</scene> serves to hold the helices in the proper orientation for binding and stabilizes the open conformation. Additionally, the [https://en.wikipedia.org/wiki/Salt_bridge_%28protein_and_supramolecular%29 salt bridges] between Glu 406, Arg 173, and Arg 346 hold the open conformation together for higher affinity (Figure 5). <ref name="Ligands">PMID: 21542831</ref> |
| - | [[Image:Screen Shot 2016-03-29 at 3.24.43 PM.png|(|):|400 px|center|thumb|'''Figure | + | [[Image:Screen Shot 2016-03-29 at 3.24.43 PM.png|(|):|400 px|center|thumb|'''Figure 5: Salt Bridge'''. The non-covalent interactions between residues Glu 406, Arg 173, and Arg 346 form a [https://en.wikipedia.org/wiki/Denticity tridentate] salt bridge. The Glu 406 acts as the central residue in the tridentate salt bridge; Arg 173 and Arg 436 both interact with Glu 406. The salt bridge is located on the intracellular side of the transmembrane helices.]] |
| Line 38: | Line 38: | ||
==Glucagon Signaling Pathway== | ==Glucagon Signaling Pathway== | ||
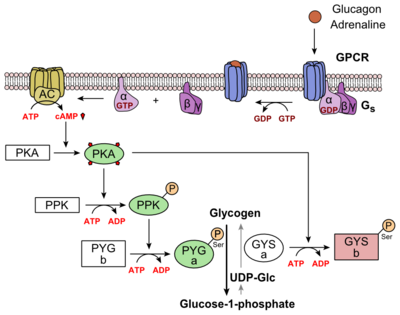
| - | Glucagon binds to the GCGR located on the [https://en.wikipedia.org/wiki/Cell_membrane plasma membrane]. Glucagon binding to GCGR induces a [https://en.wikipedia.org/wiki/Conformational_change conformational change] in GCGR. This conformation change induces the active state of the protein. The active state of the protein exchanges a [https://en.wikipedia.org/wiki/Guanosine_diphosphate guanosine diphosphate (GDP]) for [https://en.wikipedia.org/wiki/Guanosine_triphosphate guanosine triphosphate (GTP)] that is bound to the [https://en.wikipedia.org/wiki/G_alpha_subunit alpha subunit]. With the GTP in place, the activated alpha subunit dissociates from the [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G protein's]beta and gamma subunits. Following dissociation, the alpha subunit can activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase]. Activated adenylate cyclase, catalyzes the conversion of [https://en.wikipedia.org/wiki/Adenosine_triphosphate adenosine triphosphate (ATP)] into [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic adenosine monophosphate (cAMP)]. cAMP then serves as a secondary messenger to activate, through allosteric binding, [https://en.wikipedia.org/wiki/Protein_kinase_A cAMP dependent protein kinase A (PKA)]. PKA activates via [https://en.wikipedia.org/wiki/Phosphorylation phosphorylation] the [https://en.wikipedia.org/wiki/Phosphorylase_kinase phosphorylase b kinase]. The phosphorylase b kinase phosphorylates [https://en.wikipedia.org/wiki/Glycogen_phosphorylase glycogen phosphorylase b] to convert to the active form, phosphorylase a. Phosphorylase a finally catalyzes the release of [https://en.wikipedia.org/wiki/Glucose_1-phosphate glucose-1-phosphate] into the bloodstream from glycogen [https://en.wikipedia.org/wiki/Polymer polymers] (Figure | + | Glucagon binds to the GCGR located on the [https://en.wikipedia.org/wiki/Cell_membrane plasma membrane]. Glucagon binding to GCGR induces a [https://en.wikipedia.org/wiki/Conformational_change conformational change] in GCGR. This conformation change induces the active state of the protein. The active state of the protein exchanges a [https://en.wikipedia.org/wiki/Guanosine_diphosphate guanosine diphosphate (GDP]) for [https://en.wikipedia.org/wiki/Guanosine_triphosphate guanosine triphosphate (GTP)] that is bound to the [https://en.wikipedia.org/wiki/G_alpha_subunit alpha subunit]. With the GTP in place, the activated alpha subunit dissociates from the [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G protein's]beta and gamma subunits. Following dissociation, the alpha subunit can activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase]. Activated adenylate cyclase, catalyzes the conversion of [https://en.wikipedia.org/wiki/Adenosine_triphosphate adenosine triphosphate (ATP)] into [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic adenosine monophosphate (cAMP)]. cAMP then serves as a secondary messenger to activate, through allosteric binding, [https://en.wikipedia.org/wiki/Protein_kinase_A cAMP dependent protein kinase A (PKA)]. PKA activates via [https://en.wikipedia.org/wiki/Phosphorylation phosphorylation] the [https://en.wikipedia.org/wiki/Phosphorylase_kinase phosphorylase b kinase]. The phosphorylase b kinase phosphorylates [https://en.wikipedia.org/wiki/Glycogen_phosphorylase glycogen phosphorylase b] to convert to the active form, phosphorylase a. Phosphorylase a finally catalyzes the release of [https://en.wikipedia.org/wiki/Glucose_1-phosphate glucose-1-phosphate] into the bloodstream from glycogen [https://en.wikipedia.org/wiki/Polymer polymers] (Figure 6). |
| - | [[Image:Glucagon_Pathway.png|(|):|400 px|center|thumb|'''Figure | + | [[Image:Glucagon_Pathway.png|(|):|400 px|center|thumb|'''Figure 6: [https://en.wikipedia.org/wiki/Glucagon Metabolic Regulation of Glycogen by Glucagon.]'''Depicted is the visualization of the glucagon signaling pathway through the GCGR. The release of the alpha subunit from the beta and gamma subunits is depicted here, as well as the enzyme cascade to result in the releasing of glucose. Abbreviations for the enzymes in the cascade include- PPK: phosphorylase kinase; PYG b: glycogen phosphorylase b; PYG a: glycogen phosphorylase a.]] |
=Clinical Relevancy= | =Clinical Relevancy= | ||
| - | Of the fifteen human class B GPCRs, eight have been confirmed as potential [https://en.wikipedia.org/wiki/Biological_target drug target]. <ref name="Drug">PMID: 24628305</ref> | + | Of the fifteen human class B GPCRs, eight have been confirmed as potential [https://en.wikipedia.org/wiki/Biological_target drug target]. <ref name="Drug">PMID: 24628305</ref> However, overall family B GPCRS have been difficult drug targets. This difficulty is partially related to the inherent flexibility in the class B GCGR 7TM. The flexibility comes from the ability of GCGR to be a receptor many ligands. The ECD and it's role in interactions on the extracellular side of receptors may provide evidence to how class B receptors adjust the conformational spectra for various ligands. Researchers hope to show how these conformations can be utilized in potential treatments of a wide array [https://en.wikipedia.org/wiki/List_of_mental_disorders disorders]. <ref name="Drug">PMID: 24628305</ref> |
| - | + | =Potential Inhibitors= | |
| - | + | There are potential class B GCGR [https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibitors] that have clinic relevancy. These inhibitors to class B GCGRs have primarily focused on [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric inhibitors] with high specificity and the ability to treat diseases including: [https://en.wikipedia.org/wiki/Stress-related_disorders stress disorders], managing [http://www.webmd.com/diabetes/guide/diabetes-hyperglycemia hyperglycemia], and also alternative mechanisms for treating [https://en.wikipedia.org/wiki/Migraine migraines]. <ref name="Inhibitors">PMID: 24189067</ref> Inhibitors include [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibodies] which inhibit glucagon receptors through an allosteric mechanism. <ref name="Last">PMID: 19305799</ref> Further research still to be done on | |
| - | + | ==Research== | |
Determining the structure of class B GCGRs is a reason for its lack of advanced knowledge in the field, but [https://en.wikipedia.org/wiki/X-ray_crystallography X-ray crystallography] and [https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance NMR] have been the main processes performed and have had some success with it over the past couple years. <ref name="Lastt">PMID: 26227798</ref> X-ray crystallography displayed the [https://en.wikipedia.org/wiki/Crystal_structure crystal structure] of ECDs of class B GPCRs in complex with their [https://en.wikipedia.org/wiki/Ligand ligands] along with the crystal structure of the 7TM. In addition to this, NMR has allowed the ability to directly understand structures of soluble amino-terminal domains of numerous members of the secretin-like family that bind [https://en.wikipedia.org/wiki/Peptide_hormone peptide hormones]. Primary sequences analysis have led to the finding of seven segments of eighteen or more relatively hydrophobic residues that are believed to represent transmembrane helices that take part in creating an intramembranous helical bundle. <ref name="Lastt">PMID: 26227798</ref> Also, [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] has been used to determine which residues were necessary in maximizing affinity for glucagon. Finally, the orientation and mechanism of the peptide interactions within these structures are studied using peptide structure-activity relationships (SAR), receptor and ligand fragments, chimeric receptors, site-directed mutagenesis, photochemical cross-linking, and molecular modeling. <ref name="Lastt">PMID: 26227798</ref> | Determining the structure of class B GCGRs is a reason for its lack of advanced knowledge in the field, but [https://en.wikipedia.org/wiki/X-ray_crystallography X-ray crystallography] and [https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance NMR] have been the main processes performed and have had some success with it over the past couple years. <ref name="Lastt">PMID: 26227798</ref> X-ray crystallography displayed the [https://en.wikipedia.org/wiki/Crystal_structure crystal structure] of ECDs of class B GPCRs in complex with their [https://en.wikipedia.org/wiki/Ligand ligands] along with the crystal structure of the 7TM. In addition to this, NMR has allowed the ability to directly understand structures of soluble amino-terminal domains of numerous members of the secretin-like family that bind [https://en.wikipedia.org/wiki/Peptide_hormone peptide hormones]. Primary sequences analysis have led to the finding of seven segments of eighteen or more relatively hydrophobic residues that are believed to represent transmembrane helices that take part in creating an intramembranous helical bundle. <ref name="Lastt">PMID: 26227798</ref> Also, [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] has been used to determine which residues were necessary in maximizing affinity for glucagon. Finally, the orientation and mechanism of the peptide interactions within these structures are studied using peptide structure-activity relationships (SAR), receptor and ligand fragments, chimeric receptors, site-directed mutagenesis, photochemical cross-linking, and molecular modeling. <ref name="Lastt">PMID: 26227798</ref> | ||
</StructureSection> | </StructureSection> | ||
Revision as of 03:27, 19 April 2016
References
- ↑ 1.0 1.1 Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ 3.0 3.1 3.2 3.3 Yang L, Yang D, de Graaf C, Moeller A, West GM, Dharmarajan V, Wang C, Siu FY, Song G, Reedtz-Runge S, Pascal BD, Wu B, Potter CS, Zhou H, Griffin PR, Carragher B, Yang H, Wang MW, Stevens RC, Jiang H. Conformational states of the full-length glucagon receptor. Nat Commun. 2015 Jul 31;6:7859. doi: 10.1038/ncomms8859. PMID:26227798 doi:http://dx.doi.org/10.1038/ncomms8859
- ↑ 4.0 4.1 4.2 4.3 4.4 Miller LJ, Dong M, Harikumar KG. Ligand binding and activation of the secretin receptor, a prototypic family B G protein-coupled receptor. Br J Pharmacol. 2012 May;166(1):18-26. doi: 10.1111/j.1476-5381.2011.01463.x. PMID:21542831 doi:http://dx.doi.org/10.1111/j.1476-5381.2011.01463.x
- ↑ Thomsen J, Kristiansen K, Brunfeldt K, Sundby F. The amino acid sequence of human glucagon. FEBS Lett. 1972 Apr 1;21(3):315-319. PMID:11946536
- ↑ 6.0 6.1 Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, Marshall FH. Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol. 2014 Jul;171(13):3132-45. doi: 10.1111/bph.12689. PMID:24628305 doi:http://dx.doi.org/10.1111/bph.12689
- ↑ Mukund S, Shang Y, Clarke HJ, Madjidi A, Corn JE, Kates L, Kolumam G, Chiang V, Luis E, Murray J, Zhang Y, Hotzel I, Koth CM, Allan BB. Inhibitory mechanism of an allosteric antibody targeting the glucagon receptor. J Biol Chem. 2013 Nov 4. PMID:24189067 doi:http://dx.doi.org/10.1074/jbc.M113.496984
- ↑ Hoare SR. Allosteric modulators of class B G-protein-coupled receptors. Curr Neuropharmacol. 2007 Sep;5(3):168-79. doi: 10.2174/157015907781695928. PMID:19305799 doi:http://dx.doi.org/10.2174/157015907781695928