We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1165
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
=Structures of Class A vs. Class B GPCRs= | =Structures of Class A vs. Class B GPCRs= | ||
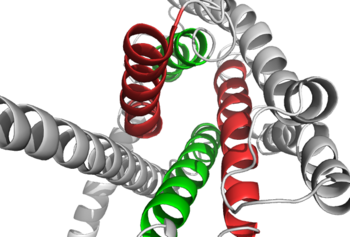
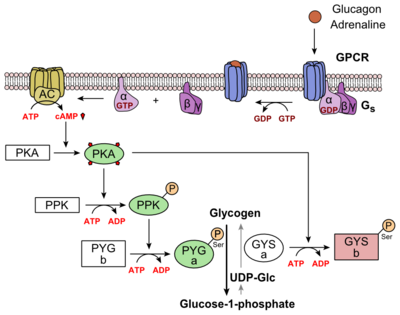
Class A vs. class B glucagon receptors share less than fifteen percent sequence homology, but both share a 7TM domain. <ref name="Intro">PMID: 24359917</ref> Understanding for class A family of GCGRs of the structure-function [https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme_Catalytic_Mechanism mechanism] has made great progress over the past few years, but understanding of class B has fallen behind but is now catching up. <ref name="Tips">PMID: 23863937</ref> Comparison of the <scene name='72/721536/Final_1st_image/1'>class B 7TM</scene> helices to that of the <scene name='72/721536/Final_class_a_7tm/1'>class A 7TM</scene> helices showed that the general orientation and positioning of the [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] are conserved through both classes. Detailed structural alignments of the two GPCR subclasses revealed multiple sequence misalignments in the transmembrane region signifying a variety of structural deviations in the transmembrane helices. <ref name="Tips">PMID: 23863937</ref> The N-terminal end of <scene name='72/721536/Final_helix_1/2'>helix one</scene> in class B GCGR, located in the 7TM, is longer than any known class A GPCR structure and stretches three supplementary helical turns above the extracellular (EC) membrane boundary. This region is referred to as the | Class A vs. class B glucagon receptors share less than fifteen percent sequence homology, but both share a 7TM domain. <ref name="Intro">PMID: 24359917</ref> Understanding for class A family of GCGRs of the structure-function [https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme_Catalytic_Mechanism mechanism] has made great progress over the past few years, but understanding of class B has fallen behind but is now catching up. <ref name="Tips">PMID: 23863937</ref> Comparison of the <scene name='72/721536/Final_1st_image/1'>class B 7TM</scene> helices to that of the <scene name='72/721536/Final_class_a_7tm/1'>class A 7TM</scene> helices showed that the general orientation and positioning of the [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] are conserved through both classes. Detailed structural alignments of the two GPCR subclasses revealed multiple sequence misalignments in the transmembrane region signifying a variety of structural deviations in the transmembrane helices. <ref name="Tips">PMID: 23863937</ref> The N-terminal end of <scene name='72/721536/Final_helix_1/2'>helix one</scene> in class B GCGR, located in the 7TM, is longer than any known class A GPCR structure and stretches three supplementary helical turns above the extracellular (EC) membrane boundary. This region is referred to as the | ||
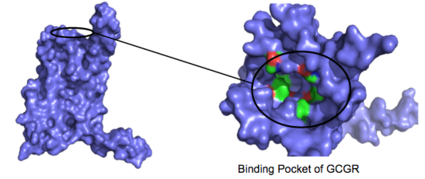
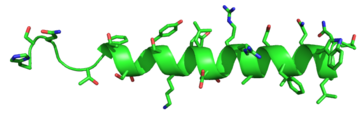
| - | <scene name='72/721535/stalk/1'>stalk</scene>. The stalk is involved in glucagon binding and helps in defining the orientation of the ECD with respect to the 7TM domain. <ref name="Tips">PMID: 23863937</ref> Also specific to class B GPCRs, a [https://en.wikipedia.org/wiki/Glycine Gly] residue at position 393 induces a <scene name='72/721535/Helical_bend/4'>bend in helix VII</scene>; this bend is stabilized by the [http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Hydrophobic_Interactions hydrophobic interaction] between the <scene name='72/721535/Gly_393_phe_184/2'> glycine 393 and phenylalanine 184</scene>. One of the most distinguishable characteristics of the class B 7TM is the <scene name='72/721536/Class_b_helix_8_tilt_finals/1'>helix VIII tilt</scene> of 25 degrees and its length compared to that of <scene name='72/721536/Class_a_helix_vii_tilt/2'>class A helix VIII tilt</scene>, which is much shorter. This helical tilt results from [https://en.wikipedia.org/wiki/Phenylalanine Glu] 406 in helix VIII that is fully conserved in secretin-like receptors and forms two interhelical [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridges] with [https://simple.wikipedia.org/wiki/Conserved_sequence conserved residues] [https://en.wikipedia.org/wiki/Arginine Arg] 173 and Arg 346. <ref name="Tips">PMID: 23863937</ref> Despite these differences, a vital region that is conserved in both class B and class A receptors is the [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin_actual/ | + | <scene name='72/721535/stalk/1'>stalk</scene>. The stalk is involved in glucagon binding and helps in defining the orientation of the ECD with respect to the 7TM domain. <ref name="Tips">PMID: 23863937</ref> Also specific to class B GPCRs, a [https://en.wikipedia.org/wiki/Glycine Gly] residue at position 393 induces a <scene name='72/721535/Helical_bend/4'>bend in helix VII</scene>; this bend is stabilized by the [http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Hydrophobic_Interactions hydrophobic interaction] between the <scene name='72/721535/Gly_393_phe_184/2'> glycine 393 and phenylalanine 184</scene>. One of the most distinguishable characteristics of the class B 7TM is the <scene name='72/721536/Class_b_helix_8_tilt_finals/1'>helix VIII tilt</scene> of 25 degrees and its length compared to that of <scene name='72/721536/Class_a_helix_vii_tilt/2'>class A helix VIII tilt</scene>, which is much shorter. This helical tilt results from [https://en.wikipedia.org/wiki/Phenylalanine Glu] 406 in helix VIII that is fully conserved in secretin-like receptors and forms two interhelical [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridges] with [https://simple.wikipedia.org/wiki/Conserved_sequence conserved residues] [https://en.wikipedia.org/wiki/Arginine Arg] 173 and Arg 346. <ref name="Tips">PMID: 23863937</ref> Despite these differences, a vital region that is conserved in both class B and class A receptors is the [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin_actual/2'>Cys 294 and Cys 224</scene> in extracellular loop two (ECL2). This bond stabilizes the receptors entire 7TM fold. Lastly, the locations of the extracellular tips for class B glucagon receptors allow for a much wider and deeper [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand-binding pocket] than any of the class A GPCRs. <ref name="Tips">PMID: 23863937</ref> These wide extracellular tip locations specifically occur between two sets of alpha helices, (Figure 1). |
| Line 31: | Line 31: | ||
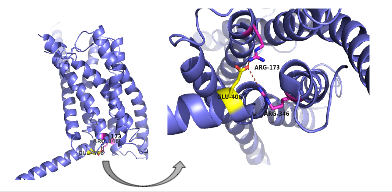
| - | There are specific amino acid interactions that maximize affinity. This includes the alpha helical structure of the <scene name='72/721535/Stalk/1'>stalk</scene>. The alpha helical structure of the stalk interacts directly with glucagon, as it extends nearly three helical turns above the membrane. When the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases. A [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] study mutating <scene name='72/721535/Ala135/1'>alanine 135</scene> to a proline. Proline disrupts helices. The Ala135Pro mutant had significant lower affinity for glucagon.<ref name="Tips">PMID: 23863937</ref> Furthermore, there are certain interactions that hold the helices of the 7TM in the conformation that maximizes [http://www.chemicool.com/definition/affinity.html affinity]. <ref name="Ligands">PMID: 21542831</ref> The high affinity conformation of GCGR is the open conformation, when glucagon can bind. Without these specific interactions between the residues, the open conformation is not stabilized and GCGR remains in the closed conformation, where glucagon cannot bind. <ref name="Tips">PMID: 23863937</ref> The [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin_actual/ | + | There are specific amino acid interactions that maximize affinity. This includes the alpha helical structure of the <scene name='72/721535/Stalk/1'>stalk</scene>. The alpha helical structure of the stalk interacts directly with glucagon, as it extends nearly three helical turns above the membrane. When the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases. A [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] study mutating <scene name='72/721535/Ala135/1'>alanine 135</scene> to a proline. Proline disrupts helices. The Ala135Pro mutant had significant lower affinity for glucagon.<ref name="Tips">PMID: 23863937</ref> Furthermore, there are certain interactions that hold the helices of the 7TM in the conformation that maximizes [http://www.chemicool.com/definition/affinity.html affinity]. <ref name="Ligands">PMID: 21542831</ref> The high affinity conformation of GCGR is the open conformation, when glucagon can bind. Without these specific interactions between the residues, the open conformation is not stabilized and GCGR remains in the closed conformation, where glucagon cannot bind. <ref name="Tips">PMID: 23863937</ref> The [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin_actual/2'>Cys 294 and Cys 224</scene> serves to hold the helices in the proper orientation for binding and stabilizes the open conformation. Additionally, the [https://en.wikipedia.org/wiki/Salt_bridge_%28protein_and_supramolecular%29 salt bridges] between |
<scene name='72/721535/Salt_bridge_residues/1'>Glu 406, Arg 173, and Arg 346</scene> hold the open conformation together for higher affinity (Figure 6). <ref name="Ligands">PMID: 21542831</ref> | <scene name='72/721535/Salt_bridge_residues/1'>Glu 406, Arg 173, and Arg 346</scene> hold the open conformation together for higher affinity (Figure 6). <ref name="Ligands">PMID: 21542831</ref> | ||
Revision as of 05:17, 19 April 2016
References
- ↑ 1.0 1.1 Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ Yang L, Yang D, de Graaf C, Moeller A, West GM, Dharmarajan V, Wang C, Siu FY, Song G, Reedtz-Runge S, Pascal BD, Wu B, Potter CS, Zhou H, Griffin PR, Carragher B, Yang H, Wang MW, Stevens RC, Jiang H. Conformational states of the full-length glucagon receptor. Nat Commun. 2015 Jul 31;6:7859. doi: 10.1038/ncomms8859. PMID:26227798 doi:http://dx.doi.org/10.1038/ncomms8859
- ↑ 4.0 4.1 4.2 4.3 4.4 Miller LJ, Dong M, Harikumar KG. Ligand binding and activation of the secretin receptor, a prototypic family B G protein-coupled receptor. Br J Pharmacol. 2012 May;166(1):18-26. doi: 10.1111/j.1476-5381.2011.01463.x. PMID:21542831 doi:http://dx.doi.org/10.1111/j.1476-5381.2011.01463.x
- ↑ Thomsen J, Kristiansen K, Brunfeldt K, Sundby F. The amino acid sequence of human glucagon. FEBS Lett. 1972 Apr 1;21(3):315-319. PMID:11946536
- ↑ 6.0 6.1 Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, Marshall FH. Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol. 2014 Jul;171(13):3132-45. doi: 10.1111/bph.12689. PMID:24628305 doi:http://dx.doi.org/10.1111/bph.12689
- ↑ Mukund S, Shang Y, Clarke HJ, Madjidi A, Corn JE, Kates L, Kolumam G, Chiang V, Luis E, Murray J, Zhang Y, Hotzel I, Koth CM, Allan BB. Inhibitory mechanism of an allosteric antibody targeting the glucagon receptor. J Biol Chem. 2013 Nov 4. PMID:24189067 doi:http://dx.doi.org/10.1074/jbc.M113.496984
- ↑ Hoare SR. Allosteric modulators of class B G-protein-coupled receptors. Curr Neuropharmacol. 2007 Sep;5(3):168-79. doi: 10.2174/157015907781695928. PMID:19305799 doi:http://dx.doi.org/10.2174/157015907781695928