We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1180
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
==Structural Considerations== | ==Structural Considerations== | ||
| - | ===Comparison between Class A and Class B GPCRs=== | ||
The class B GPCRs, of which GCGR is a member, are different from other Class A GPCRs in several ways. The first is that class B GPCRs contain a protrusion known as a 'stalk,' which is a three α-helical turn elongation of the N-terminus that protrudes past the extracellular (EC) membrane. Structural integrity of this domain in GCGR is essential to ligand binding affinity. (Fig's 1 and 2) | The class B GPCRs, of which GCGR is a member, are different from other Class A GPCRs in several ways. The first is that class B GPCRs contain a protrusion known as a 'stalk,' which is a three α-helical turn elongation of the N-terminus that protrudes past the extracellular (EC) membrane. Structural integrity of this domain in GCGR is essential to ligand binding affinity. (Fig's 1 and 2) | ||
| Line 17: | Line 16: | ||
Most notably, class B GPCRs contain a prominent central splay (Fig. 4) <scene name='72/727091/Corticotropin_glucagon_aligned/1'>(two Class B protein receptors demonstrating central splay)</scene> which is solvent filled and accessible from the extracellular side. This central splay is notably absent from class A GPCRs (Fig. 5) <scene name='72/727091/B2-adrenergic_glucagon_aligned/9'>(Class A vs. Class B GPCRs)</scene>, represents a tantalizing target for agonists/antagonists, and is the focus of much current research into GCGR signal regulation. <ref name= "Hollenstein 2014"/> | Most notably, class B GPCRs contain a prominent central splay (Fig. 4) <scene name='72/727091/Corticotropin_glucagon_aligned/1'>(two Class B protein receptors demonstrating central splay)</scene> which is solvent filled and accessible from the extracellular side. This central splay is notably absent from class A GPCRs (Fig. 5) <scene name='72/727091/B2-adrenergic_glucagon_aligned/9'>(Class A vs. Class B GPCRs)</scene>, represents a tantalizing target for agonists/antagonists, and is the focus of much current research into GCGR signal regulation. <ref name= "Hollenstein 2014"/> | ||
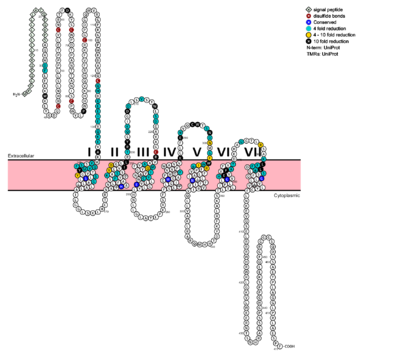
| - | ===Structurally Significant GCGR 7TDM Residues=== | ||
[[Image:Protter GLR HUMAN.png |400 px|left|thumb|Fig. 6: Snake Plot of GCGR TMD<ref name= "Siu 2013"/>]] | [[Image:Protter GLR HUMAN.png |400 px|left|thumb|Fig. 6: Snake Plot of GCGR TMD<ref name= "Siu 2013"/>]] | ||
| Line 59: | Line 57: | ||
Class B secretin-like receptors have gained relevance in therapeutics and drug targets. Maintaining information about the class B GPCRs conformational flexibility, allows for a better understanding of the receptor-ligand binding and its pharmaceutical relevance. The 7TM structure offers a direct connect between the extracellular and intracellular region, which offers a mechanism for signal transduction within the cell. GPCRs regulate cellular processes as required by the organs in which they are located. GPCR’s are used in the functioning of neuron synapses, ion transport regulation, homeostasis, cell division, and cell morphology. Mutations in the GPCR have been linked with retinitis pigmentosa, female infertility, nephrogenic diabetes insipidus, and familial exudative vitreoretinopathy. <ref name= "Salon 2011">DOI 10.1124/pr.110.003350</ref> | Class B secretin-like receptors have gained relevance in therapeutics and drug targets. Maintaining information about the class B GPCRs conformational flexibility, allows for a better understanding of the receptor-ligand binding and its pharmaceutical relevance. The 7TM structure offers a direct connect between the extracellular and intracellular region, which offers a mechanism for signal transduction within the cell. GPCRs regulate cellular processes as required by the organs in which they are located. GPCR’s are used in the functioning of neuron synapses, ion transport regulation, homeostasis, cell division, and cell morphology. Mutations in the GPCR have been linked with retinitis pigmentosa, female infertility, nephrogenic diabetes insipidus, and familial exudative vitreoretinopathy. <ref name= "Salon 2011">DOI 10.1124/pr.110.003350</ref> | ||
| - | ===Future research direction=== | ||
| - | Research for Class A GPCRs is much more extensive than for its secretin, class B counterparts, although class B is proving to be a worthwhile to invest researching. The challenge of class B stabilization, expression, and molecular size , has made class B GPCRs particularly hard to assay. Biochemical research has increased in the class B specifications, because it has been realized that receptors can be modulated by more than the agonist and antagonists present in vivo. Leading research consists of a complex interwoven scheme of equilibria manipulation in multi-receptor conformations. <ref name="Salon 2011"/> | ||
| - | |||
| - | ===Current drug targets=== | ||
A variety of small molecule modulators have been developed over the past several years providing the promise of enhanced pharmaceutical regulation of GCGR. <ref name= "Yang 2015"/>(Fig's. 12 and 13) | A variety of small molecule modulators have been developed over the past several years providing the promise of enhanced pharmaceutical regulation of GCGR. <ref name= "Yang 2015"/>(Fig's. 12 and 13) | ||
| Line 68: | Line 62: | ||
[[Image:Small molecule modulators Page 2.jpg|150 px|right|thumb|Fig. 13: Small molecule regulators of GCGR, part 2<ref name= "Yang 2015"/>.]] | [[Image:Small molecule modulators Page 2.jpg|150 px|right|thumb|Fig. 13: Small molecule regulators of GCGR, part 2<ref name= "Yang 2015"/>.]] | ||
| - | ===Possible structural considerations for large molecule agonists/antagonists=== | ||
Utilizing the visualizations of the GCGR 7TMD and glucagon peptide ligand, dimensional/structural analyses can be performed to develop models for novel molecules of increasing specificity for GCGR binding/regulation. Performing a dimensional analysis between the binding pocket and the base of the EC stalk, a large pseudopeptide molecule of 17-24 angstroms in size could be utilized to mimic the characteristics of GCGR's natural ligand, glucagon. (Fig's. 14 and 15) | Utilizing the visualizations of the GCGR 7TMD and glucagon peptide ligand, dimensional/structural analyses can be performed to develop models for novel molecules of increasing specificity for GCGR binding/regulation. Performing a dimensional analysis between the binding pocket and the base of the EC stalk, a large pseudopeptide molecule of 17-24 angstroms in size could be utilized to mimic the characteristics of GCGR's natural ligand, glucagon. (Fig's. 14 and 15) | ||
| - | |||
| - | [[Image:Movie_Frame_7.png|175 px|left|thumb|Fig. 14: Distance measurement of GCGR 7TMD Y138-D362 of 19-20 angstroms and labeled with complimentary glucagon interaction residues.]] | ||
| - | [[Image:H1___Y10_with_measurement.png|175 px|right|thumb|Fig. 15: Distance measurement of H1-Y10 of 22-24 angstroms and labeled with complimentary GCGR 7TMD residue interactions.]] | ||
==See Also== | ==See Also== | ||
| Line 78: | Line 68: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | |||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 17:59, 20 April 2016
| |||||||||||
References
- ↑ Zhang Y, Devries ME, Skolnick J. Structure modeling of all identified G protein-coupled receptors in the human genome. PLoS Comput Biol. 2006 Feb;2(2):e13. Epub 2006 Feb 17. PMID:16485037 doi:http://dx.doi.org/10.1371/journal.pcbi.0020013
- ↑ Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, Marshall FH. Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol. 2014 Jul;171(13):3132-45. doi: 10.1111/bph.12689. PMID:24628305 doi:http://dx.doi.org/10.1111/bph.12689
- ↑ 3.0 3.1 Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ Hollenstein K, Kean J, Bortolato A, Cheng RK, Dore AS, Jazayeri A, Cooke RM, Weir M, Marshall FH. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013 Jul 25;499(7459):438-43. doi: 10.1038/nature12357. Epub 2013 Jul 17. PMID:23863939 doi:http://dx.doi.org/10.1038/nature12357
- ↑ 5.0 5.1 5.2 5.3 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ 6.0 6.1 6.2 6.3 6.4 Yang DH, Zhou CH, Liu Q, Wang MW. Landmark studies on the glucagon subfamily of GPCRs: from small molecule modulators to a crystal structure. Acta Pharmacol Sin. 2015 Sep;36(9):1033-42. doi: 10.1038/aps.2015.78. Epub 2015, Aug 17. PMID:26279155 doi:http://dx.doi.org/10.1038/aps.2015.78
- ↑ Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009 May;8(5):369-85. doi: 10.1038/nrd2782. Epub 2009 Apr, 14. PMID:19365392 doi:http://dx.doi.org/10.1038/nrd2782
- ↑ 8.0 8.1 Xu Y, Xie X. Glucagon receptor mediates calcium signaling by coupling to G alpha q/11 and G alpha i/o in HEK293 cells. J Recept Signal Transduct Res. 2009 Dec;29(6):318-25. doi:, 10.3109/10799890903295150. PMID:19903011 doi:http://dx.doi.org/10.3109/10799890903295150
- ↑ Weston C, Lu J, Li N, Barkan K, Richards GO, Roberts DJ, Skerry TM, Poyner D, Pardamwar M, Reynolds CA, Dowell SJ, Willars GB, Ladds G. Modulation of Glucagon Receptor Pharmacology by Receptor Activity-modifying Protein-2 (RAMP2). J Biol Chem. 2015 Sep 18;290(38):23009-22. doi: 10.1074/jbc.M114.624601. Epub, 2015 Jul 21. PMID:26198634 doi:http://dx.doi.org/10.1074/jbc.M114.624601
- ↑ Zhang X, Stevens RC, Xu F. The importance of ligands for G protein-coupled receptor stability. Trends Biochem Sci. 2015 Feb;40(2):79-87. doi: 10.1016/j.tibs.2014.12.005. Epub, 2015 Jan 15. PMID:25601764 doi:http://dx.doi.org/10.1016/j.tibs.2014.12.005
- ↑ 'Lehninger A., Nelson D.N, & Cox M.M. (2008) Lehninger Principles of Biochemistry. W. H. Freeman, fifth edition.'
- ↑ Salon JA, Lodowski DT, Palczewski K. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol Rev. 2011 Dec;63(4):901-37. doi: 10.1124/pr.110.003350. PMID:21969326 doi:http://dx.doi.org/10.1124/pr.110.003350