We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1176
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
===Na<sup>+</sup> Binding Pocket=== | ===Na<sup>+</sup> Binding Pocket=== | ||
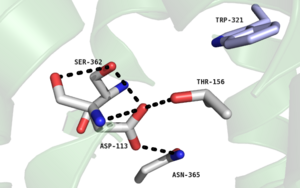
[[Image:Na+ Proteopedia Picture.png |300 px|left|thumb|Figure 2. Residues of the collapsed sodium binding pocket. Trp321 (blue) sets the top of the pocket, where Ser362, Asp113, Thr156 and Asn365 (gray) are involved in hydrogen bonding interactions preventing the coordination of a Na<sup>+</sup> ion.]] | [[Image:Na+ Proteopedia Picture.png |300 px|left|thumb|Figure 2. Residues of the collapsed sodium binding pocket. Trp321 (blue) sets the top of the pocket, where Ser362, Asp113, Thr156 and Asn365 (gray) are involved in hydrogen bonding interactions preventing the coordination of a Na<sup>+</sup> ion.]] | ||
| - | <scene name='72/721548/Trp321/1'>Trp321</scene>, which is positioned at the bottom of the <scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>, sets the top of the <scene name='72/721548/Na_bind_pocket/12'> sodium binding pocket</scene>. The Na<sup>+</sup> ion binding pocket acts as a negative allosteric site for G protein activity <ref name="SPGP"/>. When Na<sup>+</sup> enters the Na<sup>+</sup> ion binding pocket, it coordinates with Asp95, Gln131, Ser135, and Asp113, decreasing the signaling activity of NTSR1 <ref name="SPGP"/>. When NTSR1 is in its active state, the Na<sup>+</sup> ion binding pocket is collapsed | + | <scene name='72/721548/Trp321/1'>Trp321</scene>, which is positioned at the bottom of the <scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>, sets the top of the <scene name='72/721548/Na_bind_pocket/12'> sodium binding pocket</scene>. The Na<sup>+</sup> ion binding pocket acts as a negative allosteric site for G protein activity <ref name="SPGP"/>. When Na<sup>+</sup> enters the Na<sup>+</sup> ion binding pocket, it coordinates with Asp95, Gln131, Ser135, and Asp113, decreasing the signaling activity of NTSR1 <ref name="SPGP"/>. When NTSR1 is in its active state, the Na<sup>+</sup> ion binding pocket is collapsed. This prevents the regulation of protein activity through a Na<sup>+</sup> ion, as the Na<sup>+</sup> ion is unable to coordinate via a salt bridge to Asp113 (Figure 2). The side chain atoms of Asp113 form a '''[https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond]''' network with Thr156, Ser361, Ser362, and Gln365 instead, which prevents the coordination of a Na<sup>+</sup> ion<ref name ="SPGP"/> (Figure 2). |
==Activation of NTSR1== | ==Activation of NTSR1== | ||
Revision as of 00:56, 22 April 2016
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 Krumm BE, White JF, Shah P, Grisshammer R. Structural prerequisites for G-protein activation by the neurotensin receptor. Nat Commun. 2015 Jul 24;6:7895. doi: 10.1038/ncomms8895. PMID:26205105 doi:http://dx.doi.org/10.1038/ncomms8895
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ 3.0 3.1 3.2 3.3 Liang Y, Boules M, Li Z, Williams K, Miura T, Oliveros A, Richelson E. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010 Jun;58(8):1199-205. doi:, 10.1016/j.neuropharm.2010.02.015. Epub 2010 Mar 6. PMID:20211191 doi:http://dx.doi.org/10.1016/j.neuropharm.2010.02.015
- ↑ 4.0 4.1 4.2 Carraway RE, Plona AM. Involvement of neurotensin in cancer growth: evidence, mechanisms and development of diagnostic tools. Peptides. 2006 Oct;27(10):2445-60. Epub 2006 Aug 2. PMID:16887236 doi:http://dx.doi.org/10.1016/j.peptides.2006.04.030
- ↑ 5.0 5.1 5.2 Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012 May 18;11(6):462-78. doi: 10.1038/nrd3702. PMID:22596253 doi:http://dx.doi.org/10.1038/nrd3702