Introduction
Human GPR40 receptor, hGPR40, is a free fatty-acid g-protein coupled receptor that binds medium to long chain free fatty acids, inducing insulin secretion[1]. hGPR40 is highly expressed in human pancreatic β cells, brain, and endocrine cells of the gastrointestinal tract [2]. hGPR40 is of particular interest because the triggering of insulin secrection is glucose dependent.This glucose-dependence for hGPR40 signaling makes it a target for the treatment of type-2 diabetes as agonists could increase glycemic control and lower the risk of hypoglycemia[1].
Function
GPR40 is most prevalent in pancreatic β-cells where free fatty acids (FFAs) have pleiotropic effects [3]. While acute intake of FFAs stimulates insulin release, chronic exposure to high levels of FFAs results in the impairment of β-cell function and insulin secretory response [3]. GPR40 mediates the effect of both acute and chronic levels of FFAs. FFAs amplify glucose-stimulated insulin secretion from pancreatic β-cells by activating GPR40.
When GPR40 is inhibited, insulin secretion no longer increases in response to fatty acid stimulation [3]. This decreased activity of GPR40 leads to a decreased risk of hyperinsulinemia, fatty liver disease, hypertriglyceridemia, hyperglycemia, and glucose tolerance in obese patients [3]. On the contrary, overexpression of GPR40 leads to impaired β-cell function, hyperinsulinemia, and diabetes [3]. These results suggest that GPR40 plays an important role in the mechanism that links obesity and type 2 diabetes and thus is a popular drug target being studied.
Structure
hGPR40 is composed of seven-transmembrane helices that are characteristic of G-protein coupled receptors (GPCR)[1]. While there is relatively low sequence identity between hGPR40 and peptide-binding and opioid GPCRs, they do share structural similarities such as a conserved motif on (ECL2)[1]. In addition, there is a conserved that is formed between transmembrane helix 3 (Cys 79) and the C-terminus of ECL2 (Cys170)[1]. Compared to peptide-binding and opioid GPCRs which have distinctive β-sheets spanning from transmembrane helix 4 to 5, hGPR40 possesses a shorter B-sheet-like region which has low B-factors[1]. This reflects the low mobility of the region that limits the overall flexibility of the adjacent portion of ECL2 between Leu171 and Asp175[1]. A unique feature of hGPR40 is the presence of an additional 13 residues (Pro147 to Gly159) on ECL2 which is absent on all the other peptide/opioid receptors[1]. These extra residues form a separate between the B-sheet-like region and transmembrane 4. Together, the auxiliary loop and ECL2 of hGPR40 function as a over the canonical binding site covering it from the central extracellular region[1].
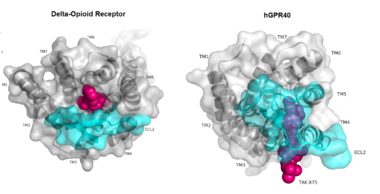
Figure 1:Comparison of Delta-opioid receptor to human free-fatty acid receptor (hGPR40) both of which are G-protein coupled receptors. The binding pocket of the delta-opioid receptor is solvent exposed allowing ligands to enter directly from the extracellular space while the binding pocket of hGPR40 is covered by the extracellular loop 2 (ECL2) preventing entry from the extracellular space (ECL2 represented in cyan). The Delta-opioid displays the canonical binding site typical of most GPCRs while ligands of hGPR40 bind to a noncanonical pocket represented in pink.
The canonical binding pocket for many other GPCRs is solvent exposed and centrally located between the transmembrane helices allowing ligands to directly bind from the extracellular space[1]. However, because the ECL2 acts as a roof to this canonical binding site, it inhibits ligands from entering directly from the extracellular region. Instead, the highly lipophilic nature of hGPRC40’s ligands allow it to enter a between TM3 and TM4 by moving through the lipid bilayer[1].
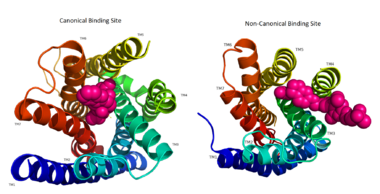
Figure 3: Comparison of the canonical binding site represented in pink of most opioid/peptide binding GPCRs (left) compared to the noncanonical binding site of ligands with hGPR40 (right).
Ligand Binding
Free Fatty Acids
GPR40’s natural substrate are FFAs in which a free carboxyl group is required to bind. However, GPR40 can be activated by a wide variety of fatty acids with chain lengths ranging from saturated fatty acids with 8 carbons to 23 carbons. In addition, various mono (i.e. palmitoleic (C16:1) and oleic (C18:1) acids) and poly-unsaturated fatty acids (i.e.linoleic (C18:2) and eicosatrienoic (C20:3) acids) can activate GPR40 [4]. The agonists potency varies according to the carbon-chain length however. The activity of GPR40 increases when the chain is increased from C6 to C15 but then decreased when the chain was extended beyond C15. One explanation for this is that as alkyl chain increased, so did the hydrophobic interactions with the protein within the binding pocket. However, for FFAs with carbon chains longer than C15, the molecular size is too large for the binding pocket. This causes the alkyl chain to extend beyond the binding pocket and destabilize the binding [5].
FFAs bind to hGPR40 by coordinating its free carboxyl group to three amino acids, , which are located close to the of hGPR40 on TM5, 6 and 7. Because of the close proximity of these residues to the extracellular domain and the dominantly hydrophobic nature of FFA’s, it is likely that ligand binding occurs close to the plane of the membrane [4].
is a partial agonist of GPR40 and tested for the treatment of type 2 diabetes. The binding of TAK-875 to hGPR40 occurs by the ligand entering the binding site through the membrane bilayer. This membrane insertion is performed via a method similar to ligand binding to sphingosine 1-phosphate receptor 1, retinal loading of GPCR opsin, and the entry of anandamide in cannabinoid receptors, in which block the binding from the extracellular matrix [6]. The binding mechanism through the bilayer may be selectively favoring the free fatty acid because of the non-polar regions of the ligand (Figure 2).
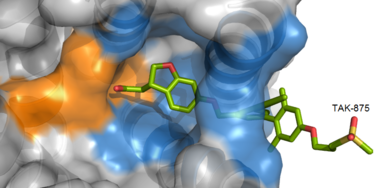
Figure 3: TAK-875 buries its polar head group within a very hydrophobic region and coordinate within the polar charge network of hGPR40.
TAK-875 binds to the created between transmembrane (TM) domains 3-5 and the extracellular loop 2 (ECL2) of hGPR40. The ECL2 and auxiliary loop form a roof causing TAK-875 to enter through TM3 and TM4, first passing through the lipid bilayer. The carboxylate of TAK-875 is buried within a very hydrophobic region and in a complex complex involving Glu172, Ser187, Asn241, and Asn 244 from hGPR40 forming ionic and polar interactions by coordinating TAK-875 with Arg183, Arg258, Tyr91, and Tyr240.
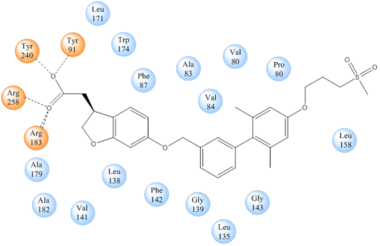
Figure 4: Stabilizing binding interactions between TAK-875 and hGPR40. Amino acids denoted in orange coordinate with the polar head of TAK-875 by polar/ionic interactions while blue amino acids stabilize the binding through hydrophobic interactions.
Signal Transduction
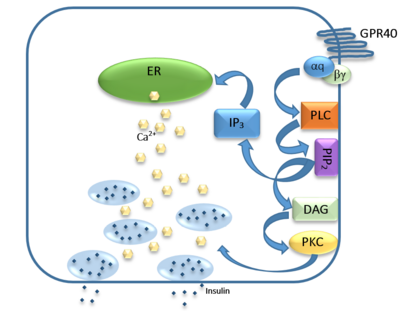
Figure 5: Signaling pathway mediated by GPR40 in pancreatic β-cells. The 𝝰q subunit represents the Gq unit that is coupled to hGPR40. This subunit breaks off to activate phospholipase C (PLC), resulting in the hydrolysis of phosphatidylinositol-4,5-biphosphate (PIP
2). PIP
2 goes on to create diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). The DAG activates protein kinase c (PKC), triggering secretion of insulin. Activated IP3 diffuses to the endoplasmic reticulum (ER), releasing the stored Ca
2+, which aids in insulin secretion.
The natural substrate of hGPR40 is free fatty acids (FFAs) which bind to the G-protein and enhance glucose-stimulated insulin secretion [4]. FFAs bind to GPR40 which then couples with the G-protein Gq leading to increased phospholipase C (PLC) activity[4]. PLC catalyzes the hydrolysis of the phospholipid phosphatidylinositol-4,5-biphosphate (PIP2) resulting in the formation of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3)[4]. DAG can then activate protein kinase C (PKC) to enhance insulin secretion[4]. IP3 on the other hand is soluble and diffuses to the endoplasmic reticulum where it binds a ligand-gated Ca2+ channel[4]. This binding triggers the opening of the channel causing stored Ca2+ to be released into the cytoplasm[4]. This large increase in intracellular free Ca2+ produces a proportional increase in glucose-dependent insulin secretion, suggesting that insulin release can be contributed in part to the changes in Ca2+ concentration resulting from activated GPR40 [4].
Medical Relevance
While undergoing clinical trials, the use of TAK-875 in the treatment of diabetes mellitus was terminated in the III phase. This was due to observed liver toxicity hypothesized to be connected to the effect of TAK-875 on bile acids, achieved through the inhibition of the efflux of bile acids into bile[7]. Other molecules are currently in development for activating hGPR40 without adversely affecting liver function. One promising lead compound is 3-ethoxypropanoic acid, however this molecule must be modified as its half-life is very short due to the rapid oxidation at the benzyl position during metabolism [8]. Additional research may hold the answer to effective treatment of type 2 diabetes and should be centered on the hGPR40 receptor because of its unique glucose dependent insulin secretion.





