User:Brittany Stankavich/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
== Structure == | == Structure == | ||
| - | hGPR40 is composed of seven-transmembrane helices that are characteristic of G-protein coupled receptors (GPCR)<ref name="crystal">PMID: 25043059</ref>. While there is relatively low sequence identity between hGPR40 and peptide-binding and opioid GPCRs, they do share structural similarities such as a conserved <scene name='72/727085/Hairpin_loop/4'>hairpin loop</scene> motif on <scene name='72/727085/Ecl2/4'>extracellular loop 2 </scene>(ECL2)<ref name="crystal"/>. In addition, there is a conserved <scene name='72/727085/Disulfide/3'>disulphide bond</scene> that is formed between transmembrane helix 3 (Cys 79) and the C-terminus of ECL2 (Cys170)<ref name="crystal"/>. Compared to peptide-binding and opioid GPCRs which have distinctive β-sheets spanning from transmembrane helix 4 to 5, hGPR40 possesses a shorter B-sheet-like region which has [http://proteopedia.org/wiki/index.php/Image:Beta-like_factors_of_hGPR40_ECL2.png low B-factors]<ref name="crystal"/>. This reflects the low mobility of the region that limits the overall flexibility of the adjacent portion of ECL2 between Leu171 and Asp175<ref name="crystal"/>. A unique feature of hGPR40 is the presence of an additional 13 residues (Pro147 to Gly159) on ECL2 which is absent on all the other peptide/opioid receptors<ref name="crystal"/>. These extra residues form a separate <scene name='72/727085/Auxiliary_loop/3'>auxiliary loop</scene> between the B-sheet-like region and transmembrane 4. Together, the auxiliary loop and ECL2 of hGPR40 function as a <scene name='72/727085/Ecl2_cap/3'>roof </scene> over the canonical binding site covering it from the central extracellular region<ref name="crystal"/>. | + | hGPR40 is composed of seven-transmembrane helices that are characteristic of [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptors] (GPCR)<ref name="crystal">PMID: 25043059</ref>. While there is relatively low sequence identity between hGPR40 and peptide-binding and [https://en.wikipedia.org/wiki/Opioid_receptor opioid GPCRs], they do share structural similarities such as a conserved <scene name='72/727085/Hairpin_loop/4'>hairpin loop</scene> motif on <scene name='72/727085/Ecl2/4'>extracellular loop 2 </scene>(ECL2)<ref name="crystal"/>. In addition, there is a conserved <scene name='72/727085/Disulfide/3'>disulphide bond</scene> that is formed between transmembrane helix 3 (Cys 79) and the C-terminus of ECL2 (Cys170)<ref name="crystal"/>. Compared to peptide-binding and opioid GPCRs which have distinctive [https://en.wikipedia.org/wiki/Beta_sheet β-sheets] spanning from transmembrane helix 4 to 5, hGPR40 possesses a shorter B-sheet-like region which has [http://proteopedia.org/wiki/index.php/Image:Beta-like_factors_of_hGPR40_ECL2.png low B-factors]<ref name="crystal"/>. This reflects the low mobility of the region that limits the overall flexibility of the adjacent portion of ECL2 between Leu171 and Asp175<ref name="crystal"/>. A unique feature of hGPR40 is the presence of an additional 13 residues (Pro147 to Gly159) on ECL2 which is absent on all the other peptide/opioid receptors<ref name="crystal"/>. These extra residues form a separate <scene name='72/727085/Auxiliary_loop/3'>auxiliary loop</scene> between the B-sheet-like region and transmembrane 4. Together, the auxiliary loop and ECL2 of hGPR40 function as a <scene name='72/727085/Ecl2_cap/3'>roof </scene> over the canonical binding site covering it from the central extracellular region<ref name="crystal"/>. |
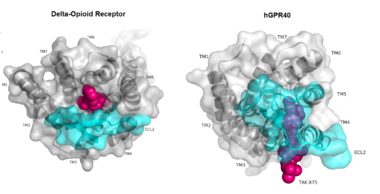
[[Image:Gpcr comparison.png|380 px|thumb|center|'''Figure 1:'''Comparison of Delta-opioid receptor to human free-fatty acid receptor (hGPR40) both of which are G-protein coupled receptors. The binding pocket of the delta-opioid receptor is solvent exposed allowing ligands to enter directly from the extracellular space while the binding pocket of hGPR40 is covered by the extracellular loop 2 (ECL2) preventing entry from the extracellular space (ECL2 represented in cyan). The Delta-opioid displays the canonical binding site typical of most GPCRs while ligands of hGPR40 bind to a noncanonical pocket represented in pink.]] | [[Image:Gpcr comparison.png|380 px|thumb|center|'''Figure 1:'''Comparison of Delta-opioid receptor to human free-fatty acid receptor (hGPR40) both of which are G-protein coupled receptors. The binding pocket of the delta-opioid receptor is solvent exposed allowing ligands to enter directly from the extracellular space while the binding pocket of hGPR40 is covered by the extracellular loop 2 (ECL2) preventing entry from the extracellular space (ECL2 represented in cyan). The Delta-opioid displays the canonical binding site typical of most GPCRs while ligands of hGPR40 bind to a noncanonical pocket represented in pink.]] | ||
| Line 34: | Line 34: | ||
=== [http://proteopedia.org/wiki/index.php/Image:Tak_875.png TAK-875] === | === [http://proteopedia.org/wiki/index.php/Image:Tak_875.png TAK-875] === | ||
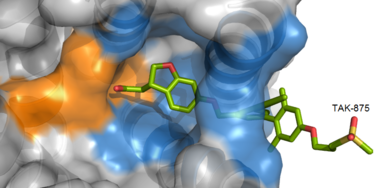
| - | <scene name='72/727085/Hgpr40_begin/3'>Tak-875</scene> is a [https://en.wikipedia.org/wiki/Partial_agonist partial agonist] of GPR40 and tested for the treatment of type 2 diabetes. The binding of TAK-875 to hGPR40 occurs by the ligand entering the binding site through the [https://en.wikipedia.org/wiki/Cell_membrane membrane bilayer]. This membrane insertion is performed via a method similar to ligand binding to [https://en.wikipedia.org/wiki/Sphingosine-1-phosphate_receptor sphingosine 1-phosphate receptor 1], retinal loading of [http://proteopedia.org/wiki/index.php/4j4q GPCR opsin], and the entry of anandamide in [https://en.wikipedia.org/wiki/Cannabinoid_receptor cannabinoid receptors], in which <scene name='72/727085/ | + | <scene name='72/727085/Hgpr40_begin/3'>Tak-875</scene> is a [https://en.wikipedia.org/wiki/Partial_agonist partial agonist] of GPR40 and tested for the treatment of type 2 diabetes. The binding of TAK-875 to hGPR40 occurs by the ligand entering the binding site through the [https://en.wikipedia.org/wiki/Cell_membrane membrane bilayer]. This membrane insertion is performed via a method similar to ligand binding to [https://en.wikipedia.org/wiki/Sphingosine-1-phosphate_receptor sphingosine 1-phosphate receptor 1], retinal loading of [http://proteopedia.org/wiki/index.php/4j4q GPCR opsin], and the entry of anandamide in [https://en.wikipedia.org/wiki/Cannabinoid_receptor cannabinoid receptors], in which the <scene name='72/727085/Ecl2/4'>extracellular loops</scene> block the binding from the extracellular matrix <ref>PMID:22344443</ref>. The binding mechanism through the bilayer may be selectively favoring the free fatty acid because of the [https://en.wikipedia.org/wiki/Chemical_polarity#Nonpolar_molecules non-polar] regions of the ligand (Figure 2). |
[[Image:Hydrophobic crop.png|380 px|center|thumb|'''Figure 3:''' TAK-875 buries its polar head group within a very hydrophobic region and coordinate within the polar charge network of hGPR40.]] | [[Image:Hydrophobic crop.png|380 px|center|thumb|'''Figure 3:''' TAK-875 buries its polar head group within a very hydrophobic region and coordinate within the polar charge network of hGPR40.]] | ||
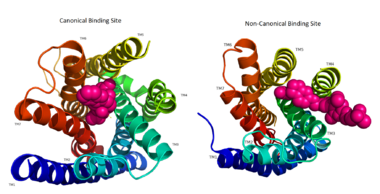
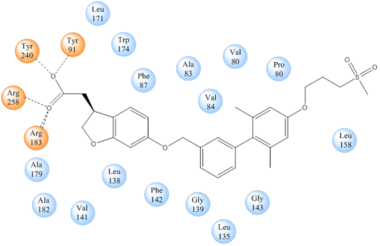
TAK-875 binds to the <scene name='72/727085/Hgpr40_entry/2'>noncanonical binding site </scene> created between transmembrane (TM) domains 3-5 and the extracellular loop 2 (ECL2) of hGPR40. The ECL2 and auxiliary loop form a roof causing TAK-875 to enter through TM3 and TM4, first passing through the lipid bilayer. The carboxylate of TAK-875 is buried within a very hydrophobic region and in a complex complex <scene name='72/727085/Hgpr40_binding_relay/6'>charge network</scene> involving Glu172, Ser187, Asn241, and Asn 244 from hGPR40 forming ionic and polar interactions by coordinating TAK-875 with Arg183, Arg258, Tyr91, and Tyr240. | TAK-875 binds to the <scene name='72/727085/Hgpr40_entry/2'>noncanonical binding site </scene> created between transmembrane (TM) domains 3-5 and the extracellular loop 2 (ECL2) of hGPR40. The ECL2 and auxiliary loop form a roof causing TAK-875 to enter through TM3 and TM4, first passing through the lipid bilayer. The carboxylate of TAK-875 is buried within a very hydrophobic region and in a complex complex <scene name='72/727085/Hgpr40_binding_relay/6'>charge network</scene> involving Glu172, Ser187, Asn241, and Asn 244 from hGPR40 forming ionic and polar interactions by coordinating TAK-875 with Arg183, Arg258, Tyr91, and Tyr240. | ||
Revision as of 16:48, 22 April 2016
- User:Brittany Stankavich/Sandbox 1
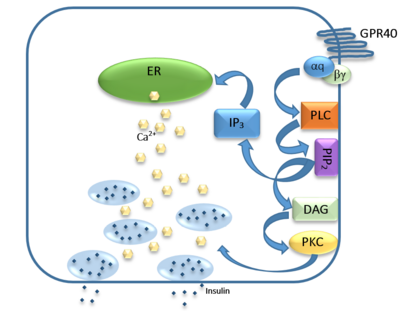
hGPR40 Homo sapiens
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, Lane W, Ivetac A, Aertgeerts K, Nguyen J, Jennings A, Okada K. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014 Jul 20. doi: 10.1038/nature13494. PMID:25043059 doi:http://dx.doi.org/10.1038/nature13494
- ↑ Ren XM, Cao LY, Zhang J, Qin WP, Yang Y, Wan B, Guo LH. Investigation of the Binding Interaction of Fatty Acids with Human G Protein-Coupled Receptor 40 Using a Site-Specific Fluorescence Probe by Flow Cytometry. Biochemistry. 2016 Mar 17. PMID:26974599 doi:http://dx.doi.org/10.1021/acs.biochem.6b00079
- ↑ 3.0 3.1 3.2 3.3 3.4 Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):82-8. doi:, 10.1016/j.prostaglandins.2009.05.003. Epub 2009 May 19. PMID:19460454 doi:http://dx.doi.org/10.1016/j.prostaglandins.2009.05.003
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Morgan NG, Dhayal S. G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic beta-cell. Biochem Pharmacol. 2009 Dec 15;78(12):1419-27. doi: 10.1016/j.bcp.2009.07.020., Epub 2009 Aug 4. PMID:19660440 doi:http://dx.doi.org/10.1016/j.bcp.2009.07.020
- ↑ Ren XM, Cao LY, Zhang J, Qin WP, Yang Y, Wan B, Guo LH. Investigation of the Binding Interaction of Fatty Acids with Human G Protein-Coupled Receptor 40 Using a Site-Specific Fluorescence Probe by Flow Cytometry. Biochemistry. 2016 Mar 17. PMID:26974599 doi:http://dx.doi.org/10.1021/acs.biochem.6b00079
- ↑ Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC. Crystal structure of a lipid G protein-coupled receptor. Science. 2012 Feb 17;335(6070):851-5. PMID:22344443 doi:10.1126/science.1215904
- ↑ Li X, Zhong K, Guo Z, Zhong D, Chen X. Fasiglifam (TAK-875) Inhibits Hepatobiliary Transporters: A Possible Factor Contributing to Fasiglifam-Induced Liver Injury. Drug Metab Dispos. 2015 Nov;43(11):1751-9. doi: 10.1124/dmd.115.064121. Epub 2015, Aug 14. PMID:26276582 doi:http://dx.doi.org/10.1124/dmd.115.064121
- ↑ Takano R, Yoshida M, Inoue M, Honda T, Nakashima R, Matsumoto K, Yano T, Ogata T, Watanabe N, Hirouchi M, Yoneyama T, Ito S, Toda N. Discovery of DS-1558: A Potent and Orally Bioavailable GPR40 Agonist. ACS Med Chem Lett. 2015 Jan 13;6(3):266-70. doi: 10.1021/ml500391n. eCollection, 2015 Mar 12. PMID:25815144 doi:http://dx.doi.org/10.1021/ml500391n