Sandbox WWC11
From Proteopedia
| Line 10: | Line 10: | ||
---- | ---- | ||
| - | + | '''Introduction''' | |
| - | + | ||
| Line 18: | Line 17: | ||
This protein is composed of two identical monomers linked by a cystine bridge. The total molecular weight of this protein is 146kDa. These two monomers can work independently to each other and consist of a light and heavy amino acid chain. The active site on the protein consists of a deep crevice where a heme molecule is covalently attached. The deep pocket is believe to limit access to the heme and increase specificity for smaller molecules to be oxidized. This pocket also possesses a hydrophobic pocket for stabilization of substrate.<ref name="Active Site Structure">PMID:16288920</ref> | This protein is composed of two identical monomers linked by a cystine bridge. The total molecular weight of this protein is 146kDa. These two monomers can work independently to each other and consist of a light and heavy amino acid chain. The active site on the protein consists of a deep crevice where a heme molecule is covalently attached. The deep pocket is believe to limit access to the heme and increase specificity for smaller molecules to be oxidized. This pocket also possesses a hydrophobic pocket for stabilization of substrate.<ref name="Active Site Structure">PMID:16288920</ref> | ||
| - | A lack of or mutation in this enzyme can cause immune deficiency, although most individuals with this mutation seem to be able to compensate with other, still unknown mechanisms. Over activation of myloperoxidase has been linked to increased inflammation and is being studied in relation to inceased risk of cardiovascular disease.<ref name="Association between myeloperoxidase levels and risk of coronary artery disease">PMID:11694155</ref> There are still many unknown surrounding the complete function and role that myeloperoxidase plays in the body's immune response. However, it has become clear that this protein is an integral piece in the | + | A lack of or mutation in this enzyme can cause immune deficiency, although most individuals with this mutation seem to be able to compensate with other, still unknown mechanisms. Over activation of myloperoxidase has been linked to increased inflammation and is being studied in relation to inceased risk of cardiovascular disease.<ref name="Association between myeloperoxidase levels and risk of coronary artery disease">PMID:11694155</ref> There are still many unknown surrounding the complete function and role that myeloperoxidase plays in the body's immune response. However, it has become clear that this protein is an integral piece in the immune response and warrants further research. |
| + | |||
| + | '''Structure''' | ||
| + | |||
| + | |||
| + | '''Function''' | ||
| + | |||
| + | |||
| + | '''Implication of Potential Defects or Deficiencies''' | ||
| + | |||
| + | |||
| + | '''Correlation to Cardiovascular Disease''' | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
==3D structures of myeloperoxidase== | ==3D structures of myeloperoxidase== | ||
| + | |||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
Revision as of 00:34, 26 April 2016
| |||||||||
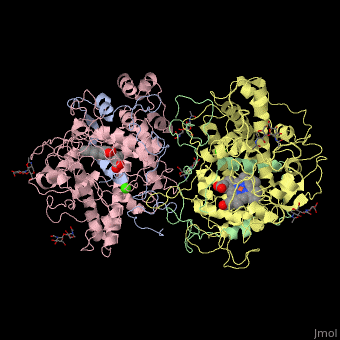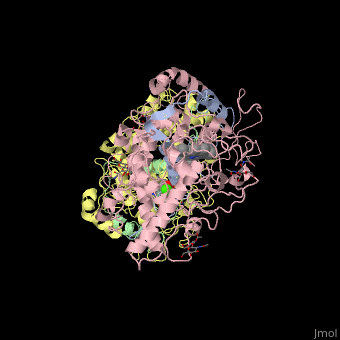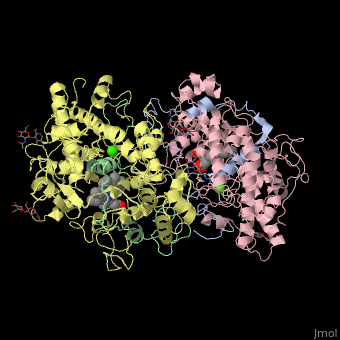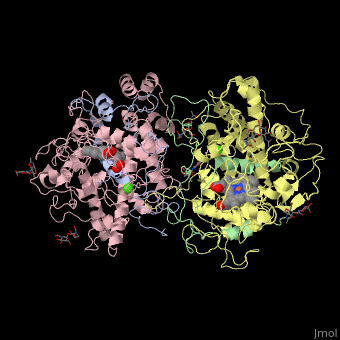
| Dog glycosylated myeloperoxidase dimer: heavy chain (pink and yellow), light chain (grey and green) containing heme complex with Ca+2 ions (green), 1d5l | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | Peroxidase, with EC number 1.11.1.7 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Myeloperoxidase
Introduction
Myeloperoxidase (MPO) is a protein found in nertrophil, the most common white blood cell. This enzyme generates hypochlorous acid (HOCl) from hydrogen peroxide in addition to other hypohalous acids depending on what is available in the cell. The HOCl is then used, in combination with other molecules, in an oxidative burst that kills bacteria and other potentially harmful invaders one taken up by the cell. This protein falls into a group of heme peroxidase enzymes. The role of these enzymes is to oxidize molecules using the heme[1].
This protein is composed of two identical monomers linked by a cystine bridge. The total molecular weight of this protein is 146kDa. These two monomers can work independently to each other and consist of a light and heavy amino acid chain. The active site on the protein consists of a deep crevice where a heme molecule is covalently attached. The deep pocket is believe to limit access to the heme and increase specificity for smaller molecules to be oxidized. This pocket also possesses a hydrophobic pocket for stabilization of substrate.[2]
A lack of or mutation in this enzyme can cause immune deficiency, although most individuals with this mutation seem to be able to compensate with other, still unknown mechanisms. Over activation of myloperoxidase has been linked to increased inflammation and is being studied in relation to inceased risk of cardiovascular disease.[3] There are still many unknown surrounding the complete function and role that myeloperoxidase plays in the body's immune response. However, it has become clear that this protein is an integral piece in the immune response and warrants further research.
Structure
Function
Implication of Potential Defects or Deficiencies
Correlation to Cardiovascular Disease
3D structures of myeloperoxidase
Updated on 26-April-2016
1myp – MPO – dog
1mhl, 1cxp – hMPO C – human
3f9p - hMPO
1qo4 - MPO – Arabidopsis thaliana
1d2v – hMPO C + Br
1d5l – hMPO + CN
1d7w - hMPO + CN + Br
1dnu - hMPO + SCN
1dnw - hMPO + CN + SCN
3zs0, 3zs1, 4c1m - hMPO + inhibitor
4dl1 - hMPO + thioxanthine
4ejx - hMPO + ceruplasmin
Problem fixed, India! You needed to include the references section of the page. It doesn't know where to link the citations without this!
References
- ↑ Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998 Nov 1;92(9):3007-17. PMID:9787133
- ↑ Yin J, Bergmann EM, Cherney MM, Lall MS, Jain RP, Vederas JC, James MN. Dual modes of modification of hepatitis A virus 3C protease by a serine-derived beta-lactone: selective crystallization and formation of a functional catalytic triad in the active site. J Mol Biol. 2005 Dec 9;354(4):854-71. Epub 2005 Oct 14. PMID:16288920 doi:10.1016/j.jmb.2005.09.074
- ↑ Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001 Nov 7;286(17):2136-42. PMID:11694155