Nos1
From Proteopedia
| Line 2: | Line 2: | ||
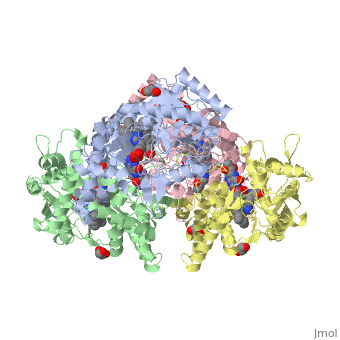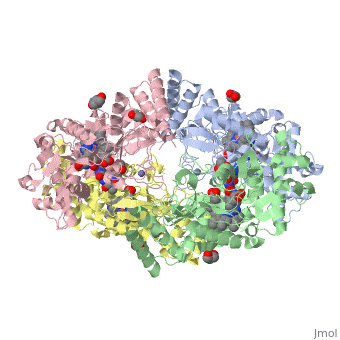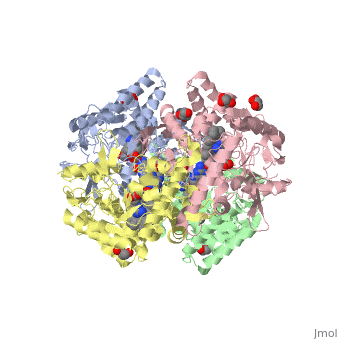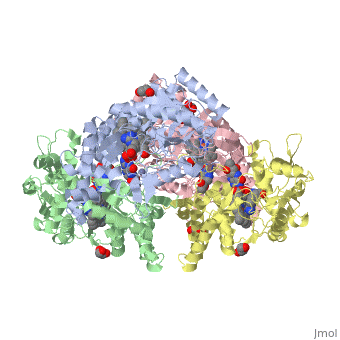
<StructureSection load='4D1N' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='4D1N' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | + | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | |
== Location == | == Location == | ||
| Line 13: | Line 13: | ||
== Function == | == Function == | ||
| - | NOS1 is a major component of the production of Nitric Oxide | + | NOS1 is a major component of the production of Nitric Oxide. Nitric Oxide targets NO receptors on G-cylcase and other Nitric Oxide specific receptors as well. NOS1 is an important component of synaptogenesis, long-term potentiation, neurotransmitter release and synaptic plasticity <ref>Juliane Kopf, Martin Schecklmann, Tim Hahn, Thomas Dresler, Alica C. Dieler, Martin J. Herrmann, Andreas J. Fallgatter, Andreas Reif. (2011) NOS1 ex1f-VNTR polymorphism influences prefrontal brain oxygenation during a working memory task, NeuroImage, Volume 57, (Issue 4),1617-1623, Ihttp://dx.doi.org/10.1016/</ref>.NOS1 is coupled with N-methyl-D-Aspartate (NMDA) receptors in the post-synapse of cells, which allows these processes to occur. The NMDA receptor binds to PSD95, which than binds to NOS1. After the complex is formed, NDMDA receptor mediated CA2+ influx now regulates the amount of Nitric Oxide that is produced by NOS1 <ref>Freudenberg, F., Alttoa, A. & Reif, A. (2015) Neuronal nitric oxide synthase (NOS1) and its adaptor (NOS1AP) act as a genetic risk factors for psychiatric. Genes Brain Behav 14, 47–64.</ref>. |
| - | NOS1 also contributes in limiting | + | NOS1 also contributes in limiting oxidative stress in the myocardium, limits systolic and diastolic dysfunction and prevent a failing heart. NOS1 regulates the reuptake of Ca2+ in sarcoplamisic reticulum (SR). NOS1 is shown into the human coronary artery smooth cells and maintains of basal blood flow. NOS1 also controls the parasympathetic and sympathetic regulation of the heart<ref>Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]</ref>. NOS1 is important in all functions that help regulate the cardiac muscles<ref>Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]</ref>. The ability of Nitric Oxide to self regulate enables NOS1 to control all these major functions. In myocite relaxation, the Nitric Oxide produced by NOS1 facilitates SERCA to increase intracellular CA2+ because of the increase in PKA dependent PLN phosphorylation. NOS1 also regulates cardiac function by the Nitric Oxide produced targets that are not CA2+ dependent<ref>Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]</ref>. |
== Disease == | == Disease == | ||
| Line 20: | Line 20: | ||
Single nucleotide polymorphisms (SNPs) and various lengths of tandem repeats within NOS1 have been linked in other disorders of the brain such as Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Out of three identified SNPs occurring in alternative exon 1c, only the SNP G-84A has a functional effect that decreases transcription levels (Galimberti, et. al, 2008). However, various lengths of tandem repeats present in the alternative exon 1f has been shown to be a potential factor in both Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Shorter tandem TG repeats are possibly associated with the development of Alzheimer’s disease, and longer tandem TG repeats are possibly associated with the development of Parkinson’s disease (Rife, et. al, 2009). Although schizophrenia, Alzheimer’s, and Parkinson’s diseases have genetic influences, mutations in NOS1 can be a risk indicator for developing these diseases (Shinkai, et. al, 2002; Galimberti, et. al, 2008; Rife, et. al, 2009). | Single nucleotide polymorphisms (SNPs) and various lengths of tandem repeats within NOS1 have been linked in other disorders of the brain such as Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Out of three identified SNPs occurring in alternative exon 1c, only the SNP G-84A has a functional effect that decreases transcription levels (Galimberti, et. al, 2008). However, various lengths of tandem repeats present in the alternative exon 1f has been shown to be a potential factor in both Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Shorter tandem TG repeats are possibly associated with the development of Alzheimer’s disease, and longer tandem TG repeats are possibly associated with the development of Parkinson’s disease (Rife, et. al, 2009). Although schizophrenia, Alzheimer’s, and Parkinson’s diseases have genetic influences, mutations in NOS1 can be a risk indicator for developing these diseases (Shinkai, et. al, 2002; Galimberti, et. al, 2008; Rife, et. al, 2009). | ||
| + | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
Revision as of 16:50, 27 April 2016
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
Shinkai, T., Ohmori, O., Hori, H., and Nakamura, J. (2002) Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Molecular Psychiatry. 7, 560-563. doi:10.1038/sj.mp.4001041 Galimberti, D., Scarpini, E., Venturelli, E., Strobel, A., Herterich, S., Fenogolio, C., Guidi, I., Scalabrini, D., Cortini, F., Bresolin, N., Lesch, K., and Reif, A. (2008) Association of a NOS1 promoter repeat with Alzheimer’s disease. Neurobiology of Aging. 29, 1359-1365. doi:10.1016/j.neurobiolaging.2007.03.003 Rife, T., Rasoul, B., Pullen, N., Mitchell, D., Grathwol, K., and Kurth, J. (2009) The effect of a promoter polymorphism on transcription of nitric oxide synthase 1 and its relevance to Parkinson’s disease. Journal of Neuroscience Research. 87, 2319-2325. doi:10.1005/jnr.22045
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ UniProt Consortium 2009, ‘UniProtKB - P29475 (NOS1_HUMAN),’ UniProtKB Protein Knowledgebase
- ↑ Ward ME, Toporsian M, Scott JA, et al. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. Journal of Clinical Investigation. 2005;115(11):3128-3139. doi:10.1172/JCI20806.
- ↑ Juliane Kopf, Martin Schecklmann, Tim Hahn, Thomas Dresler, Alica C. Dieler, Martin J. Herrmann, Andreas J. Fallgatter, Andreas Reif. (2011) NOS1 ex1f-VNTR polymorphism influences prefrontal brain oxygenation during a working memory task, NeuroImage, Volume 57, (Issue 4),1617-1623, Ihttp://dx.doi.org/10.1016/
- ↑ Freudenberg, F., Alttoa, A. & Reif, A. (2015) Neuronal nitric oxide synthase (NOS1) and its adaptor (NOS1AP) act as a genetic risk factors for psychiatric. Genes Brain Behav 14, 47–64.
- ↑ Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]
- ↑ Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]
- ↑ Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]