Nos1
From Proteopedia
| Line 19: | Line 19: | ||
Nitric oxide (NO) is produced from one of three synthases present within the body: neuronal nitric oxide synthase 1 (NOS1), inducible nitric oxide synthase 2 (NOS2), and endothelial nitric oxide synthase 3 (NOS3) (Shinkai, et. al, 2002). Specifically, NOS1 has nine different first exons that lead to multiple NOS1 transcripts with different 5’-untranslated regions (Galimberti, et. al, 2008). An advantage of having multiple first exons that can be alternatively spliced and expressed is that NOS1 can be specific and specifically regulated for different tissues (Galimberti, et. al, 2008). A disadvantage to having multiple first exons is that there is a higher possibility of mutations, which would affect NO production and thus have an effect on the second messenger cyclic guanine monophosphate (cGMP) production (Shinkai, et. al, 2002). Furthermore, because NO is an oxyradical, overproduction caused by a mutation can lead to neural tissue damage (Shinkai, et. al, 2002). Numerous pathologies may arise from neural tissue damage, and one study suggested overproduction of NO that leads to such damage is possibly an influential factor in developing schizophrenia (Shinkai, et. al, 2002). | Nitric oxide (NO) is produced from one of three synthases present within the body: neuronal nitric oxide synthase 1 (NOS1), inducible nitric oxide synthase 2 (NOS2), and endothelial nitric oxide synthase 3 (NOS3) (Shinkai, et. al, 2002). Specifically, NOS1 has nine different first exons that lead to multiple NOS1 transcripts with different 5’-untranslated regions (Galimberti, et. al, 2008). An advantage of having multiple first exons that can be alternatively spliced and expressed is that NOS1 can be specific and specifically regulated for different tissues (Galimberti, et. al, 2008). A disadvantage to having multiple first exons is that there is a higher possibility of mutations, which would affect NO production and thus have an effect on the second messenger cyclic guanine monophosphate (cGMP) production (Shinkai, et. al, 2002). Furthermore, because NO is an oxyradical, overproduction caused by a mutation can lead to neural tissue damage (Shinkai, et. al, 2002). Numerous pathologies may arise from neural tissue damage, and one study suggested overproduction of NO that leads to such damage is possibly an influential factor in developing schizophrenia (Shinkai, et. al, 2002). | ||
Single nucleotide polymorphisms (SNPs) and various lengths of tandem repeats within NOS1 have been linked in other disorders of the brain such as Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Out of three identified SNPs occurring in alternative exon 1c, only the SNP G-84A has a functional effect that decreases transcription levels (Galimberti, et. al, 2008). However, various lengths of tandem repeats present in the alternative exon 1f has been shown to be a potential factor in both Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Shorter tandem TG repeats are possibly associated with the development of Alzheimer’s disease, and longer tandem TG repeats are possibly associated with the development of Parkinson’s disease (Rife, et. al, 2009). Although schizophrenia, Alzheimer’s, and Parkinson’s diseases have genetic influences, mutations in NOS1 can be a risk indicator for developing these diseases (Shinkai, et. al, 2002; Galimberti, et. al, 2008; Rife, et. al, 2009). | Single nucleotide polymorphisms (SNPs) and various lengths of tandem repeats within NOS1 have been linked in other disorders of the brain such as Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Out of three identified SNPs occurring in alternative exon 1c, only the SNP G-84A has a functional effect that decreases transcription levels (Galimberti, et. al, 2008). However, various lengths of tandem repeats present in the alternative exon 1f has been shown to be a potential factor in both Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Shorter tandem TG repeats are possibly associated with the development of Alzheimer’s disease, and longer tandem TG repeats are possibly associated with the development of Parkinson’s disease (Rife, et. al, 2009). Although schizophrenia, Alzheimer’s, and Parkinson’s diseases have genetic influences, mutations in NOS1 can be a risk indicator for developing these diseases (Shinkai, et. al, 2002; Galimberti, et. al, 2008; Rife, et. al, 2009). | ||
| - | |||
| - | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
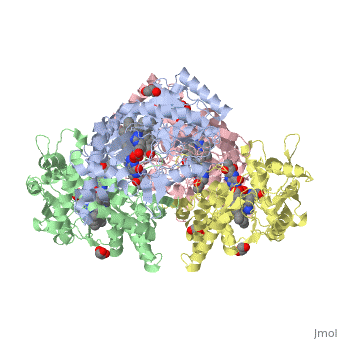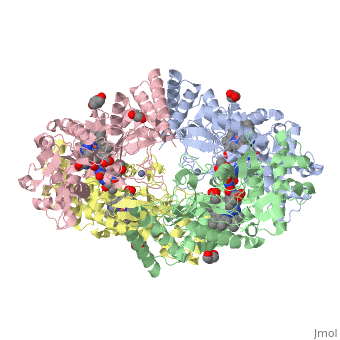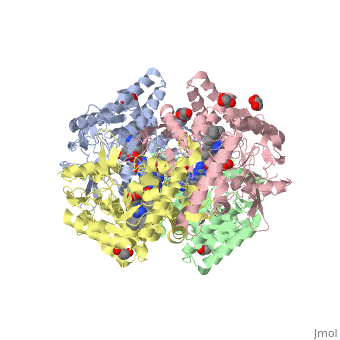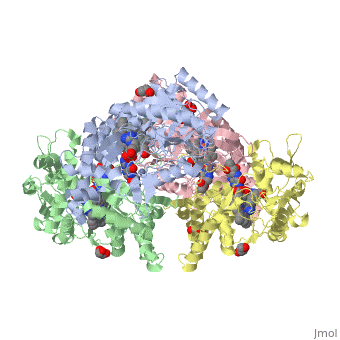
| - | Nos exists as a homodimer. This homodimer consists of two regions. One region is an N-terminal oxygenase domain and the other is a C-terminal reductase. The N-terminal oxygenase domain is an extended beta sheet cage with binding sites for heme and pterin. | + | Nos exists as a homodimer. This homodimer consists of two regions. One region is an N-terminal oxygenase domain and the other is a C-terminal reductase. The N-terminal oxygenase domain is an extended beta sheet cage with binding sites for heme and pterin.The N-terminal catalytic domain contains a heme active site, a nearby cofactor site for tetrahydrobiopterin (H4B) and a C-terminal reductase domain that consists of FMN, FAD, and NADPH binding sites. The structure of the human nNOS heme domain contains cysteine and tryptophan. The NADPH provides electrons for catalysis. These electrons are first passed to FAD and FMN and then the heme. This electron flow is monitored by the binding of CaM and Calcium2+ in the linker region between the two heterodimer domains. |
| + | The amino acid composition of asparagine and methionine in rat neuronal NOS are conserved in human nNos. This amino acid composition influences the binding of inhibitors and makes nNos inhibitors highly selective. The structure of the human nNOS heme domain contains cysteine and tryptophan. The cysteine subunits of one region pack closely against the backbone of histidine in subunits of another region. Crystal packing interactions most likely contribute to the fully ordered N-termini in the NOS structure | ||
| + | There is a flexible surface loop downstream from the Zn2+ binding site. This loop in human nNOS lacks any secondary structure and appears as a random coil. This reflects the flexible nature of nNOS because it can easily adapt to local environment which involves crystal packing. This loop is found near the CaM binding site and can therefore most likely interact with it and affect electron flow. | ||
| Line 33: | Line 33: | ||
Rife, T., Rasoul, B., Pullen, N., Mitchell, D., Grathwol, K., and Kurth, J. (2009) The effect of a promoter polymorphism on transcription of nitric oxide synthase 1 and its relevance to Parkinson’s disease. Journal of Neuroscience Research. 87, 2319-2325. doi:10.1005/jnr.22045 | Rife, T., Rasoul, B., Pullen, N., Mitchell, D., Grathwol, K., and Kurth, J. (2009) The effect of a promoter polymorphism on transcription of nitric oxide synthase 1 and its relevance to Parkinson’s disease. Journal of Neuroscience Research. 87, 2319-2325. doi:10.1005/jnr.22045 | ||
<references/> | <references/> | ||
| + | Li H, Jamal J, Plaza C, et al. Structures of human constitutive nitric oxide synthases. Acta Crystallographica Section D: Biological Crystallography. 2014;70(Pt 10):2667-2674. | ||
Revision as of 23:24, 27 April 2016
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
Shinkai, T., Ohmori, O., Hori, H., and Nakamura, J. (2002) Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Molecular Psychiatry. 7, 560-563. doi:10.1038/sj.mp.4001041 Galimberti, D., Scarpini, E., Venturelli, E., Strobel, A., Herterich, S., Fenogolio, C., Guidi, I., Scalabrini, D., Cortini, F., Bresolin, N., Lesch, K., and Reif, A. (2008) Association of a NOS1 promoter repeat with Alzheimer’s disease. Neurobiology of Aging. 29, 1359-1365. doi:10.1016/j.neurobiolaging.2007.03.003 Rife, T., Rasoul, B., Pullen, N., Mitchell, D., Grathwol, K., and Kurth, J. (2009) The effect of a promoter polymorphism on transcription of nitric oxide synthase 1 and its relevance to Parkinson’s disease. Journal of Neuroscience Research. 87, 2319-2325. doi:10.1005/jnr.22045
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ UniProt Consortium 2009, ‘UniProtKB - P29475 (NOS1_HUMAN),’ UniProtKB Protein Knowledgebase
- ↑ Ward ME, Toporsian M, Scott JA, et al. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. Journal of Clinical Investigation. 2005;115(11):3128-3139. doi:10.1172/JCI20806.
- ↑ Juliane Kopf, Martin Schecklmann, Tim Hahn, Thomas Dresler, Alica C. Dieler, Martin J. Herrmann, Andreas J. Fallgatter, Andreas Reif. (2011) NOS1 ex1f-VNTR polymorphism influences prefrontal brain oxygenation during a working memory task, NeuroImage, Volume 57, (Issue 4),1617-1623, Ihttp://dx.doi.org/10.1016/
- ↑ Freudenberg, F., Alttoa, A. & Reif, A. (2015) Neuronal nitric oxide synthase (NOS1) and its adaptor (NOS1AP) act as a genetic risk factors for psychiatric. Genes Brain Behav 14, 47–64.
- ↑ Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]
- ↑ Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]
- ↑ Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]
Li H, Jamal J, Plaza C, et al. Structures of human constitutive nitric oxide synthases. Acta Crystallographica Section D: Biological Crystallography. 2014;70(Pt 10):2667-2674.