Sandbox WWC7
From Proteopedia
| Line 12: | Line 12: | ||
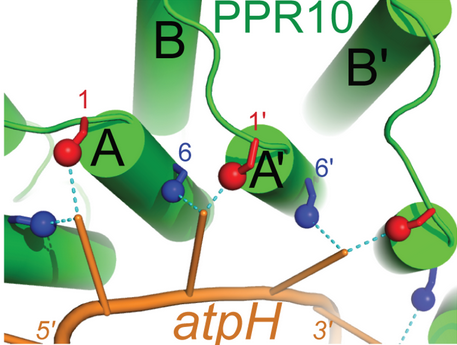
This image shows the general code by which PPR proteins recognize and bind RNA in a modular fashion<ref name = "barkan"/> | This image shows the general code by which PPR proteins recognize and bind RNA in a modular fashion<ref name = "barkan"/> | ||
| - | While crystallographic structures show PPR10 binding RNA in a dimerized configuration, further evidence by [ | + | While crystallographic structures show PPR10 binding RNA in a dimerized configuration, further evidence by <span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0021967309003057 EC-SY-SAX]</span> has shown that this result is likely an artifact of the high concentrations necessary for crystallography. In a natural setting, PPR10 does not form a dimer.<ref name = "gully">DOI:10.1093/nar/gkv027</ref><ref name = "li">DOI:10.1074/jbc.M114.575472</ref> |
===RNA Stabilization=== | ===RNA Stabilization=== | ||
Revision as of 15:50, 9 May 2016
Pentatricopeptide repeat (PPR) proteins are a family of sequence specific RNA-binding proteins which participate in organelle RNA metabolism. Although the mechanisms of RNA binding and the functions of PPR proteins are not fully understood, PPR proteins are thought to assist in RNA editing,[1] translation,[2] and organelle biogenesis.[3] While PPR proteins are found in many eukaryotes, they make up the majority of RNA-binding factors in plant organelles. PPR proteins are characterized by a series of tandem-repeat amino acid consensus sequences which form α-helix . These hairpin structures accumulate to form an (blue to red from N terminus to C terminus). PPR proteins belong to one of two classes: P-class and PLS-class, with the P-class containing 35 amino acid repeats and the PLS-class containing 31-36 amino acid repeats. PPR10 (shown to the right dimerized and bound to RNA) is a well-characterized P-class PPR protein found in the chloroplast of Zea mays which is often used as a model PPR protein.[2]
|
Contents |
Function
In the Zea mays plastid, PPR10 binds specifically to the ssRNA oligonucleotides atpH (17 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') and (18 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') where it has been shown to prevent degradation of sequences both upstream and downstream of its binding sites. In addition to stabilizing these RNA sequences, PPR10 increases the rate at which they are translated.[2]
Mechanism
The primary factor in the ability of PPR10 to bind RNA bases in a modular fashion lies in the identities of the residue at position 6 on a repeat and the residue at position 1 on the next repeat (designated 1'). For example, in the structure to the right, Through van der Waals interactions, Val210 and Arg175 (both orange) also contribute to the specific binding of guanine in this example. These residues force G1 into a conformation where it forms a hydrogen bond to Thr178. The example of PPR10 binding G1 of spaJ exemplifies the general rules by which PPR proteins bind specific nucleotides: firstly, a residue at the 6 position of one repeat (Thr178 in the previous example) forms a hydrogen bond with the base. The identity of this residue determines whether the repeat will bind a purine (adenine and guanine) or pyrimidine (cytosine and uracil). It appears that serine and threonine at position 6 are specific for purines, and asparagine at position 6 is specific for pyrimidines.[4] Secondly, a residue at position 1' (Val210 in the previous example) completes the specificity of the interaction. Through van der Waals interactions, this residue determines between A/G and C/U. Other amino acids further contribute to this mechanism, but the previously described rules always apply when PPR proteins bind RNA sequences with modularity.[5]
This image shows the general code by which PPR proteins recognize and bind RNA in a modular fashion[4]
While crystallographic structures show PPR10 binding RNA in a dimerized configuration, further evidence by EC-SY-SAX has shown that this result is likely an artifact of the high concentrations necessary for crystallography. In a natural setting, PPR10 does not form a dimer.[6][7]
RNA Stabilization
PPR10 stabilizes RNA upstream and downstream of its binding sites by blocking exonucleases from degrading RNA in either the 5' or 3' direction.[2]
Translation Enhancement
The mechanism by which PPR10 increases the rate of translation is still unknown. However, it is predicted that PPR10 binds to sequences near the ribosome binding site of the RNA transcript. By doing so, PPR10 prevents the ~20 nucleotides to which it is bound from base pairing to the ribosome binding site and impairing translation. Considering the bacterial origins of the chloroplast, it is interesting to note that the RNA stabilizing and translation enhancing properties of PPR10 in the platid mirror the some of the functions of small RNAs (smRNA) in bacteria. In bacterial cells, smRNAs similarly bind regions near ribosome binding sites of mRNA, preventing degradation by nucleases and increasing translation by preventing base-pairing to ribosome binding sites.[2]
Synthetic Applications
Recent advances in programmable site-directed DNA-binding proteins (such as CRISPR/Cas9) have shown incredible potential for medical, agricultural, and scientific application. As a result, it is not surprising that
References
- ↑ Okuda K, Nakamura T, Sugita M, Shimizu T, Shikanai T. A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J Biol Chem. 2006 Dec 8;281(49):37661-7. Epub 2006 Oct 2. PMID:17015439 doi:http://dx.doi.org/10.1074/jbc.M608184200
- ↑ 2.0 2.1 2.2 2.3 2.4 Prikryl J, Rojas M, Schuster G, Barkan A. Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein. Proc Natl Acad Sci U S A. 2011 Jan 4;108(1):415-20. doi: 10.1073/pnas.1012076108., Epub 2010 Dec 20. PMID:21173259 doi:http://dx.doi.org/10.1073/pnas.1012076108
- ↑ Lurin C, Andres C, Aubourg S, Bellaoui M, Bitton F, Bruyere C, Caboche M, Debast C, Gualberto J, Hoffmann B, Lecharny A, Le Ret M, Martin-Magniette ML, Mireau H, Peeters N, Renou JP, Szurek B, Taconnat L, Small I. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004 Aug;16(8):2089-103. Epub 2004 Jul 21. PMID:15269332 doi:http://dx.doi.org/10.1105/tpc.104.022236
- ↑ 4.0 4.1 Barkan A, Rojas M, Fujii S, Yap A, Chong YS, Bond CS, Small I. A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet. 2012;8(8):e1002910. doi: 10.1371/journal.pgen.1002910. Epub 2012 Aug , 16. PMID:22916040 doi:http://dx.doi.org/10.1371/journal.pgen.1002910
- ↑ Yagi Y, Nakamura T, Small I. The potential for manipulating RNA with pentatricopeptide repeat proteins. Plant J. 2014 Jun;78(5):772-82. doi: 10.1111/tpj.12377. Epub 2014 Jan 29. PMID:24471963 doi:http://dx.doi.org/10.1111/tpj.12377
- ↑ Gully BS, Cowieson N, Stanley WA, Shearston K, Small ID, Barkan A, Bond CS. The solution structure of the pentatricopeptide repeat protein PPR10 upon binding atpH RNA. Nucleic Acids Res. 2015 Feb 18;43(3):1918-26. doi: 10.1093/nar/gkv027. Epub 2015 , Jan 21. PMID:25609698 doi:http://dx.doi.org/10.1093/nar/gkv027
- ↑ Li Q, Yan C, Xu H, Wang Z, Long J, Li W, Wu J, Yin P, Yan N. Examination of the Dimerization States of the Single-stranded RNA-recognition Protein PPR10. J Biol Chem. 2014 Sep 17. pii: jbc.M114.575472. PMID:25231995 doi:http://dx.doi.org/10.1074/jbc.M114.575472