User:R. Jeremy Johnson/Neurotensin
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
==Neurotensin Receptor (NTSR1)== | ==Neurotensin Receptor (NTSR1)== | ||
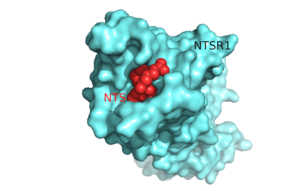
| - | [[Image:Surface_Protein_red.png |300 px|below|thumb|'''Figure 1'''.Top view of NTSR1 protein (blue) interacting with its ligand, NTS(red).]] | ||
== Introduction == | == Introduction == | ||
| - | The neurotensin receptor (NTSR1) belongs to the superfamily of proteins known as [http://proteopedia.org/wiki/index.php/G_protein-coupled_receptor G protein-coupled receptors] (GPCRs) and responds to the 13 amino acid hormone [https://en.wikipedia.org/wiki/Neurotensin neurotensin (NTS)]. Currently around 800 G protein-coupled receptors have been identified and are hypothesized to be responsible for roughly 80% of [https://en.wikipedia.org/wiki/Signal_transduction signal transduction].<ref name="Millar">PMID:20019124</ref> GPCRs are involved in a vast array of physiological processes within the body that range from interactions with [https://en.wikipedia.org/wiki/Dopamine dopamine] to effects on secretion of bile in the intestines.<ref name="Gui">PMID:11208724</ref> <ref name="Binder">PMID:1173461</ref> Due to the vast array of functions that these proteins serve and their high abundance within the body, these proteins have become major drug targets.<ref name="Fang">PMID:23573662</ref> | + | [[Image:Surface_Protein_red.png |300 px|below|thumb|'''Figure 1'''.Top view of NTSR1 protein (blue) interacting with its ligand, NTS(red).]] The neurotensin receptor (NTSR1) belongs to the superfamily of proteins known as [http://proteopedia.org/wiki/index.php/G_protein-coupled_receptor G protein-coupled receptors] (GPCRs) and responds to the 13 amino acid hormone [https://en.wikipedia.org/wiki/Neurotensin neurotensin (NTS)]. Currently around 800 G protein-coupled receptors have been identified and are hypothesized to be responsible for roughly 80% of [https://en.wikipedia.org/wiki/Signal_transduction signal transduction].<ref name="Millar">PMID:20019124</ref> GPCRs are involved in a vast array of physiological processes within the body that range from interactions with [https://en.wikipedia.org/wiki/Dopamine dopamine] to effects on secretion of bile in the intestines.<ref name="Gui">PMID:11208724</ref> <ref name="Binder">PMID:1173461</ref> Due to the vast array of functions that these proteins serve and their high abundance within the body, these proteins have become major drug targets.<ref name="Fang">PMID:23573662</ref> |
The ligand for NTSR1 is the 13 amino acid peptide, neurotensin (NTS)<ref name="SONT">PMID:23051748</ref>, and the majority of the effects of NTS are mediated through NTSR1<ref name="SONT"/>. NTS has a variety of biological activities including a role in the '''[https://en.wikipedia.org/wiki/Leptin leptin]''' signaling pathways <ref name="Mice">PMID: 20211191</ref>, tumor growth <ref name="cancer">PMID:16887236</ref>, and '''[https://en.wikipedia.org/wiki/Dopamine dopamine]''' regulation <ref name="Schizophrenia">PMID:22596253</ref>. NTSR1 was crystallized bound with a C-terminal portion of its tridecapeptide '''[https://en.wikipedia.org/wiki/Ligand ligand]''', <scene name='72/721548/Neurotensin/7'>NTS(8-13)</scene>. The shortened ligand was used because it has a higher potency and efficacy than its full-length counterpart<ref name="SONT"/>. | The ligand for NTSR1 is the 13 amino acid peptide, neurotensin (NTS)<ref name="SONT">PMID:23051748</ref>, and the majority of the effects of NTS are mediated through NTSR1<ref name="SONT"/>. NTS has a variety of biological activities including a role in the '''[https://en.wikipedia.org/wiki/Leptin leptin]''' signaling pathways <ref name="Mice">PMID: 20211191</ref>, tumor growth <ref name="cancer">PMID:16887236</ref>, and '''[https://en.wikipedia.org/wiki/Dopamine dopamine]''' regulation <ref name="Schizophrenia">PMID:22596253</ref>. NTSR1 was crystallized bound with a C-terminal portion of its tridecapeptide '''[https://en.wikipedia.org/wiki/Ligand ligand]''', <scene name='72/721548/Neurotensin/7'>NTS(8-13)</scene>. The shortened ligand was used because it has a higher potency and efficacy than its full-length counterpart<ref name="SONT"/>. | ||
| Line 26: | Line 25: | ||
<scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene>. <ref name="SONT"/>Binding of NTS to the binding site on NTSR1 is enriched by <scene name='72/721539/Binding_pocket_surface/4'>charge complementarity</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)]between the positive NTS arginine side chains and the [https://en.wikipedia.org/wiki/Electronegativity electronegative] pocket. The protein is colored by charge: <font color='#FF0000'>negative</font> and <font color='#0000CD'>positive</font>. Two of NTS's arginine residues are colored <font color='#0000CD'>blue</font>. In addition, the C-terminus of <font color='#32CD32'>NTS</font> forms a <scene name='72/721539/Binding_site_charges/4'>salt bridge</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] with Arg328 of <font color='#A9A9A9'>NTSR1</font>. Three [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds] are made between the side chains of NTS and the receptor while most of the interactions are a result of [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] interactions. The binding pocket is partially capped by a <scene name='72/721539/B-hairpin_loop/1'>Β-hairpin loop</scene> at the proximal end of the receptor's N-terminus.<ref name="White"/> The interactions in the binding site cause a wide spread conformational change in the receptor leading to the receptor to adopt an active conformation. This active conformation allows for the activation of the associated intracellular G-protein. | <scene name='72/721547/Hydrophobic_binding_pocket/6'>hydrophobic binding pocket</scene>. <ref name="SONT"/>Binding of NTS to the binding site on NTSR1 is enriched by <scene name='72/721539/Binding_pocket_surface/4'>charge complementarity</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)]between the positive NTS arginine side chains and the [https://en.wikipedia.org/wiki/Electronegativity electronegative] pocket. The protein is colored by charge: <font color='#FF0000'>negative</font> and <font color='#0000CD'>positive</font>. Two of NTS's arginine residues are colored <font color='#0000CD'>blue</font>. In addition, the C-terminus of <font color='#32CD32'>NTS</font> forms a <scene name='72/721539/Binding_site_charges/4'>salt bridge</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] with Arg328 of <font color='#A9A9A9'>NTSR1</font>. Three [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds] are made between the side chains of NTS and the receptor while most of the interactions are a result of [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] interactions. The binding pocket is partially capped by a <scene name='72/721539/B-hairpin_loop/1'>Β-hairpin loop</scene> at the proximal end of the receptor's N-terminus.<ref name="White"/> The interactions in the binding site cause a wide spread conformational change in the receptor leading to the receptor to adopt an active conformation. This active conformation allows for the activation of the associated intracellular G-protein. | ||
| - | The binding of NTS to NTSR1 is also driven by hydrophobic stacking. One key residue in this pocket is a Phenylalanine at position 358, which takes part in a network of hydrophobic stacking interactions<ref name="SPGP"/>. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the | + | The binding of NTS to NTSR1 is also driven by hydrophobic stacking. One key residue in this pocket is a Phenylalanine at position 358, which takes part in a network of hydrophobic stacking interactions<ref name="SPGP"/>. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the [https://en.wikipedia.org/wiki/C-terminus C-terminal] <scene name='72/721547/Ligand_protein_interactions/8'>Leu13 residue of NTS ligand</scene> via [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions].<ref name="SONT"/><ref name="SPGP"/> Without the hydrophobic stacking interactions that are facilitated by the Phe358, this binding interaction would be destabilized. Trp321 also participates in these stacking interactions and serves as the boundary between the ligand binding pocket and the Na<sup>+</sup> binding pocket.<ref name="SPGP"/> Another major player in the transduction of the extracellular signal to the intracellular G-protein is the <scene name='72/727765/Hydrogen_bonding_network/4'>hydrogen bonding network</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)]that links the bound <font color='#32CD32'>hormone</font> with the [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] core of the <font color='#A9A9A9'>neurotensin receptor</font>. The carboxylate of Leu13 of NTS forms a hydrogen bond network with R327, R328, and Y324. The Tyr324, in turn, is brought into an orientation to make the formation of a <scene name='72/727765/Hydrophobic_stacking_4xee/2'>hydrophobic stacking</scene> (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE]) network between F358, W321, A157, and F317 possible.<ref name="Krumm">PMID:23051748</ref> The effects that this network has on the activation of the intracellular G-protein was examined by the [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] of amino acids that disrupted the formation of this network. Mutagenesis of <scene name='72/727765/Overall_structure/12'>A86L</scene>, <scene name='72/727765/Overall_structure/13'>E166A</scene>, <scene name='72/727765/Overall_structure/14'>G125L</scene>, <scene name='72/727765/Overall_structure/15'>L310A</scene>, <scene name='72/727765/Overall_structure/16'>F358A</scene>, and <scene name='72/727765/Overall_structure/17'>V360A</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] showed that when this interaction was disrupted, the receptor no longer was able to activate the G-protein. <ref name="Krumm"/> This discovery lead to the conclusion that the conformational changes caused by this stacking allows for the signal to be moved from the extracellular binding site through the transmembrane helices of the receptor to the intracellular region activating the G-protein. |
| - | <scene name='72/721547/Ligand_protein_interactions/8'>Leu13 residue of NTS ligand</scene> via | + | |
===Na<sup>+</sup> Binding Pocket=== | ===Na<sup>+</sup> Binding Pocket=== | ||
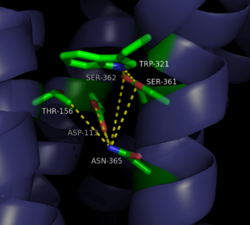
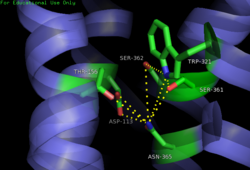
| - | [[Image:4XEE closed sodium pocket.png| | + | [[Image:4XEE closed sodium pocket.png|250 px|right|thumb|Figure 3: Closed form of sodium binding pocket that caps the entrance of sodium into the top of the binding pocket. (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE])]] |
| - | [[Image:4GRV open binding pocket.png| | + | [[Image:4GRV open binding pocket.png|250 px|right|thumb|Figure 4: Open form of sodium binding pocket that does not cap the entrance of sodium into the top of the binding pocket. (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV])]] Conserved across all class A GPCRs, a <scene name='72/727765/Sodium_binding_pocket_final/1'>sodium binding pocket</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV]) is seen in the middle of TM2 helix. The sodium ion is coordinated with a highly conserved Asp113 and four other oxygen contacts from a combination of water molecules. For G-protein activation to be possible, a hydrogen bond coordination with T156, S362, and N365 of the NPxxY [https://en.wikipedia.org/wiki/Structural_motif motif] must occur. Trp321 helps to maintain the active conformation of the receptor by occluding the top of the binding pocket using [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] interactions (Figure 2). This occlusion stops sodium ions from entering the top of the binding pocket and helps NTSR1 remain in its active conformation. The conformation of the binding pocket where Trp321 does not occlude the top can be seen when mutations to A86L, G215A, and V360A are present (Figure 3). This form of the receptor would allow more sodium into the binding pocket and therefor stabilize the inactive receptor form. <ref name="Katritch">PMID:24767681</ref> <scene name='72/721548/Trp321/1'>Trp321</scene>, which is positioned at the bottom of the <scene name='72/721547/Hydrophobic_binding_pocket/5'>hydrophobic binding pocket</scene>, sets the top of the <scene name='72/721548/Na_bind_pocket/12'> sodium binding pocket</scene>. The Na<sup>+</sup> ion binding pocket acts as a negative allosteric site for G protein activity <ref name="SPGP"/>. When Na<sup>+</sup> enters the Na<sup>+</sup> ion binding pocket, it coordinates with Asp95, Gln131, Ser135, and Asp113, decreasing the signaling activity of NTSR1 <ref name="SPGP"/>. When NTSR1 is in its active state, the Na<sup>+</sup> ion binding pocket is collapsed. This prevents the regulation of protein activity through a Na<sup>+</sup> ion, as the Na<sup>+</sup> ion is unable to coordinate via a salt bridge to Asp113 (Figure 2). The side chain atoms of Asp113 form a '''[https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond]''' network with Thr156, Ser361, Ser362, and Gln365 instead, which prevents the coordination of a Na<sup>+</sup> ion<ref name ="SPGP"/> (Figure 2). |
Sodium ions are a negative [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric] inhibitor to the binding of the neurotensin [https://en.wikipedia.org/wiki/Agonist agonist] to the binding site on the neurotensin receptor. Sodium's binding causes for the receptor to favor its inactive state by disrupting the hydrogen bond network between nearby amino acids. This disruption in the hydrogen bond network causes the pocket to be in its uncollapsed form. Asp113 of the highly conserved D/RY motif and Asn365 of the highly conserved NPxxY motif form a substantial hydrogen bonding network with T156 and S362.<ref name="Krumm"/> This hydrogen bonding network prevents the incorporation of the sodium ion by collapsing upon itself and filling the sodium binding pocket. Trp321 also works to inhibit the incorporation of the sodium ion by capping off the sodium binding pocket to not allow sodium to enter from the top. Trp321 uses Van der Waals interactions to place it in the conformation necessary to block sodium from entering the site. By not allowing for sodium to enter this binding site, the receptor is able to conform to its active state and activate the G-protein that is associated with it. | Sodium ions are a negative [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric] inhibitor to the binding of the neurotensin [https://en.wikipedia.org/wiki/Agonist agonist] to the binding site on the neurotensin receptor. Sodium's binding causes for the receptor to favor its inactive state by disrupting the hydrogen bond network between nearby amino acids. This disruption in the hydrogen bond network causes the pocket to be in its uncollapsed form. Asp113 of the highly conserved D/RY motif and Asn365 of the highly conserved NPxxY motif form a substantial hydrogen bonding network with T156 and S362.<ref name="Krumm"/> This hydrogen bonding network prevents the incorporation of the sodium ion by collapsing upon itself and filling the sodium binding pocket. Trp321 also works to inhibit the incorporation of the sodium ion by capping off the sodium binding pocket to not allow sodium to enter from the top. Trp321 uses Van der Waals interactions to place it in the conformation necessary to block sodium from entering the site. By not allowing for sodium to enter this binding site, the receptor is able to conform to its active state and activate the G-protein that is associated with it. | ||
| Line 62: | Line 60: | ||
===Cancer Studies=== | ===Cancer Studies=== | ||
Some tumor cells can secrete and express NTS and NTS receptors themselves suggesting that NTS '''[https://en.wikipedia.org/wiki/Autocrine_signalling autocrine]''', '''[https://en.wikipedia.org/wiki/Endocrine_system endocrine]''' and '''[https://en.wikipedia.org/wiki/Paracrine_signalling paracrine]''' regulation are possible. This leads to aggressive growth and tumor development. Injecting animals with NTS increased tumor growth and size, while injecting them with NTS antagonist had the opposite effect <ref name="cancer"/>. NTS regulation may be used in future cancer treatments. | Some tumor cells can secrete and express NTS and NTS receptors themselves suggesting that NTS '''[https://en.wikipedia.org/wiki/Autocrine_signalling autocrine]''', '''[https://en.wikipedia.org/wiki/Endocrine_system endocrine]''' and '''[https://en.wikipedia.org/wiki/Paracrine_signalling paracrine]''' regulation are possible. This leads to aggressive growth and tumor development. Injecting animals with NTS increased tumor growth and size, while injecting them with NTS antagonist had the opposite effect <ref name="cancer"/>. NTS regulation may be used in future cancer treatments. | ||
| - | + | ||
===Dopamine Regulation=== | ===Dopamine Regulation=== | ||
The '''[http://www.schizophreniaforum.org/for/curr/AbiDargham/ dopamine hypothesis]''' states that hyperdopamine levels may lead to schizophrenic symptoms. NTSR1 causes a blockade which inhibits firing in dopaminergic cells suggesting that NTSR1 could be used in schizophrenia treatment. However, this led to extreme secondary effects and was discontinued. Despite this, research on NTSR1 as a treatment for schizophrenia persists<ref name="Schizophrenia"/>. | The '''[http://www.schizophreniaforum.org/for/curr/AbiDargham/ dopamine hypothesis]''' states that hyperdopamine levels may lead to schizophrenic symptoms. NTSR1 causes a blockade which inhibits firing in dopaminergic cells suggesting that NTSR1 could be used in schizophrenia treatment. However, this led to extreme secondary effects and was discontinued. Despite this, research on NTSR1 as a treatment for schizophrenia persists<ref name="Schizophrenia"/>. | ||
===Clinical Relevance=== | ===Clinical Relevance=== | ||
| - | NTSR1 is commonly expressed in various invasive [https://en.wikipedia.org/wiki/Cancer cancer] cell lines making it a promising cancer drug target. It is prevalent in [https://en.wikipedia.org/wiki/Colorectal_cancer colon cancer] [https://en.wikipedia.org/wiki/Adenocarcinoma adenocarcinoma], but is not found in adult colon cell types.<ref name="Valerie">PMID:21903767</ref> NTSR1 is also found in aggressive [https://en.wikipedia.org/wiki/Prostate_cancer prostate cancer] cells, but not [https://en.wikipedia.org/wiki/Epithelium epithelial] prostate cells. In prostate cancer cells, binding of NTS results in [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase mitogen-activated protein kinase (PKB)], [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase phosphoinositide-3 kinase (PI-3K)], [https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor epidermal growth factor receptor (EGFR)], [https://en.wikipedia.org/wiki/Proto-oncogene_tyrosine-protein_kinase_Src SRC], and [https://en.wikipedia.org/wiki/STAT5 STAT5] phosphorylation.<ref name="Valerie"/> These all result in increased DNA synthesis, [https://en.wikipedia.org/wiki/Cell_growth cell proliferation], and survival. Inhibition of NTSR1 and its downstream signaling represents a target for [https://en.wikipedia.org/wiki/Radiation_therapy radiotherapy], which uses radiation to target malignant cells. NTSR1 can be inhibited by agonist [https://en.wikipedia.org/wiki/Meclinertant meclinertant] which inhibits proliferation and prosurvival of cancer cells. Combination treatment of radiation and meclinerant provides selective treatment of cancer cells over normal cells, indicating the need for clinical trials of this approach. <ref name="Kisfalvi">PMID:19679549</ref> | + | [[Image: Meclinerant.jpg |200 px|right|thumb|Figure 5: Meclinerant: An inhibitor of NTSR1 found to enhance selectivity of radiotherapy in cancer treatment (PubMed).]] NTSR1 is commonly expressed in various invasive [https://en.wikipedia.org/wiki/Cancer cancer] cell lines making it a promising cancer drug target. It is prevalent in [https://en.wikipedia.org/wiki/Colorectal_cancer colon cancer] [https://en.wikipedia.org/wiki/Adenocarcinoma adenocarcinoma], but is not found in adult colon cell types.<ref name="Valerie">PMID:21903767</ref> NTSR1 is also found in aggressive [https://en.wikipedia.org/wiki/Prostate_cancer prostate cancer] cells, but not [https://en.wikipedia.org/wiki/Epithelium epithelial] prostate cells. In prostate cancer cells, binding of NTS results in [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase mitogen-activated protein kinase (PKB)], [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase phosphoinositide-3 kinase (PI-3K)], [https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor epidermal growth factor receptor (EGFR)], [https://en.wikipedia.org/wiki/Proto-oncogene_tyrosine-protein_kinase_Src SRC], and [https://en.wikipedia.org/wiki/STAT5 STAT5] phosphorylation.<ref name="Valerie"/> These all result in increased DNA synthesis, [https://en.wikipedia.org/wiki/Cell_growth cell proliferation], and survival. Inhibition of NTSR1 and its downstream signaling represents a target for [https://en.wikipedia.org/wiki/Radiation_therapy radiotherapy], which uses radiation to target malignant cells. NTSR1 can be inhibited by agonist [https://en.wikipedia.org/wiki/Meclinertant meclinertant] which inhibits proliferation and prosurvival of cancer cells. Combination treatment of radiation and meclinerant provides selective treatment of cancer cells over normal cells, indicating the need for clinical trials of this approach. <ref name="Kisfalvi">PMID:19679549</ref> |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 19:31, 20 May 2016
References
- ↑ Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010 Jan;24(1):261-74. Epub 2009 Dec 17. PMID:20019124 doi:10.1210/me.2009-0473
- ↑ Gui X, Carraway RE. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology. 2001 Jan;120(1):151-60. PMID:11208724
- ↑ Selivonenko VG. [The interrelationship between electrolytes and phase analysis of systole in toxic goiter]. Probl Endokrinol (Mosk). 1975 Jan-Feb;21(1):19-23. PMID:1173461
- ↑ Fang Y, Lahiri J, Picard L. G protein-coupled receptor microarrays for drug discovery. Drug Discov Today. 2004 Dec 15;9(24 Suppl):S61-7. PMID:23573662
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ 6.0 6.1 6.2 6.3 Liang Y, Boules M, Li Z, Williams K, Miura T, Oliveros A, Richelson E. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010 Jun;58(8):1199-205. doi:, 10.1016/j.neuropharm.2010.02.015. Epub 2010 Mar 6. PMID:20211191 doi:http://dx.doi.org/10.1016/j.neuropharm.2010.02.015
- ↑ 7.0 7.1 7.2 Carraway RE, Plona AM. Involvement of neurotensin in cancer growth: evidence, mechanisms and development of diagnostic tools. Peptides. 2006 Oct;27(10):2445-60. Epub 2006 Aug 2. PMID:16887236 doi:http://dx.doi.org/10.1016/j.peptides.2006.04.030
- ↑ 8.0 8.1 8.2 Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012 May 18;11(6):462-78. doi: 10.1038/nrd3702. PMID:22596253 doi:http://dx.doi.org/10.1038/nrd3702
- ↑ 9.0 9.1 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854-61. PMID:4745447
- ↑ Kitabgi P. Neurotensin modulates dopamine neurotransmission at several levels along brain dopaminergic pathways. Neurochem Int. 1989;14(2):111-9. PMID:20504406
- ↑ Mustain WC, Rychahou PG, Evers BM. The role of neurotensin in physiologic and pathologic processes. Curr Opin Endocrinol Diabetes Obes. 2011 Feb;18(1):75-82. doi:, 10.1097/MED.0b013e3283419052. PMID:21124211 doi:http://dx.doi.org/10.1097/MED.0b013e3283419052
- ↑ Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999 Jul;20(7):302-9. PMID:10390649
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 14.13 14.14 14.15 14.16 14.17 14.18 14.19 14.20 14.21 Krumm BE, White JF, Shah P, Grisshammer R. Structural prerequisites for G-protein activation by the neurotensin receptor. Nat Commun. 2015 Jul 24;6:7895. doi: 10.1038/ncomms8895. PMID:26205105 doi:http://dx.doi.org/10.1038/ncomms8895
- ↑ 15.0 15.1 15.2 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ 17.0 17.1 Valerie NC, Casarez EV, Dasilva JO, Dunlap-Brown ME, Parsons SJ, Amorino GP, Dziegielewski J. Inhibition of neurotensin receptor 1 selectively sensitizes prostate cancer to ionizing radiation. Cancer Res. 2011 Nov 1;71(21):6817-26. doi: 10.1158/0008-5472.CAN-11-1646. Epub, 2011 Sep 8. PMID:21903767 doi:http://dx.doi.org/10.1158/0008-5472.CAN-11-1646
- ↑ Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009 Aug 15;69(16):6539-45. doi: 10.1158/0008-5472.CAN-09-0418. PMID:19679549 doi:http://dx.doi.org/10.1158/0008-5472.CAN-09-0418