metabotropic Glutamate Receptor 5
Introduction
Receiving and responding to extracellular messages is critical to the proper function of the nervous system. Glutamate is the primary excitory neurotransmitter of the Central Nervous System (CNS), and metabotropic glutamate receptor 5 is a key member of the glutamate signaling pathway.[1] Metabotropic glutamate receptor 5 is a homodimeric GPCR that resides in the cellular membrane [1]. mGlu5 is a member of the Class C GPCR family and can further be categorized into the Group I subgroup [2].
The functionality of the mGlu5 receptor is determined by conformational changes throughout multiple domains. mGlu5 will bind glutamate through its extracellular Venus flytrap domain and the signal will be transduced across the membrane to a heterotrimeric G protein, which will ultimately lead to calcium release and the activation of PKC[2]. The signal is relayed through a Gq/11 pathway[1]. Activated PKC will elicit a excitory post-synaptic response and modulate long term potentiation in the CNS[2].
Human mGlu5 is found throughout the central nervous system. Areas containing high concentrations of mGlu5 are often involved in emotional processing and higher cognition.[3] The localization of mGlu5 in the CNS and the presence of multiple domains makes mGlu5 an excellent target for treating neurological conditions including: schizophrenia, Fragile X, [1], anxiety, and Alzheimer's disease[2].
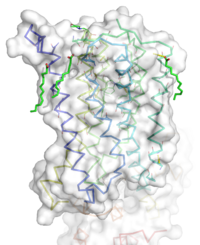
Figure 1: Overall Structure of the mGlu5 TMD. The polar heads on the Oleic acids orient the image with the top oriented extracellularly, the middle portion inserted into the membrane, and the lower portion oriented intracellularly. The white exterior represents the surface of the protein, and the multicolored lines interior to the surface represent the backbones 7 transmembrane alpha helices.
Discovery
The mGlu family of receptors was the first of the Class C GPCR to be extensively studied[2]. The first regions of the protein crystallized and studied were the Venus Fly Trap domain and the cysteine -rich domain (CRD) on the extracellular region of the receptor[1].The Venus Fly Trap domain is a large extracellular domain that will selectively bind to glutamate[2]. The CRD is a somewhat smaller domain composed of many ß sheets and cysteine residues [2]. The CRD acts as a signal mediator between the Venus Flytrap domain and the transmembrane domain (TMD) of mGlu5, by linking to each domain with disulfide bonds[2]. The hydrophobic nature and flexibility of the mGlu5 TMD made it difficult to crystallize.
Recently, the human metabotropic glutamate receptor 5 transmembrane domain was crystallized and a structure elucidated[1]. Several modifications were made to the TMD for successful crystallization. The protein was thermostabilized and flexible domains were removed[1]. In total residues 2-568 and 837-1153 were excised from the structure. These flexible domains allow mGlu5 to bind to its GPCR [1]. The of the alpha helices are shown in green, and the negative allosteric modulator mavoglurant shown in yellow. Also in orange, a T4 -Lysozyme was inserted into intracellular loop 2 to add stability[1].
Structure
Overall Structure
The TMD contains 7 that spans the membrane. The protein was crystallized with shown in red. On the top portion of the protein several critical extracellular loops connect the TMD to the CRD. The binding pocket can be found deep in the interior of the protein, and is mainly comprised of hydrophobic amino acids with more polar amino acids found in the upper and lower portions of the binding site[1]. Inserted into the biding pocket is the negative allosteric modulator mavoglurant. The TMD is in an inactive conformation, since mavoglurant is bound[1]. Also, the deletion of the flexible domains leaves the mGlu5 receptor unable to bind to its GPCR[1]. The inactive state is maintained by multiple ionic locks whose positions determine the active versus inactive conformation.
A number of intramolecular interactions within the trans-membrane domain stabilize the inactive conformation of mGlu5, as demonstrated by being represented in the inactivate state. While in the inactive state, glutamate binding to mGlu5 triggers a conformational change that leads to mGlu5 being in the active state and hence initiates the aforementioned Gq pathway. The first of these interactions is an ionic interaction, termed the , between Lysine 665 of TM3 and Glutamate 770 of TM6. Evidence for the importance of this interaction came through a kinetic study of mutant proteins where both residues were separately substituted with alanine, resulting in constitutive activity of the GPCR and its coupled pathway.[1] A second critical interaction that stabilizes the inactive conformer is a between Serine 614 of ICL1 and Arginine 668 of TM3. Similarly, when Serine 614 was substituted with alanine, high levels of activity were seen in the mutant GPCR.[1] A between Cysteine 644 of TM3 and Cysteine 733 of is critical at anchoring ECL2 and is highly conserved across Class C GPCR’s.[1] The ECL2's presence combined with the helical bundle of the trans-membrane domain creates a that restricts entrance to the allosteric binding site within the seven trans-membrane α-helices. This restricted entrance has no effect on the natural ligand, glutamate, as glutamate binds to the extracellular domain, but this entrance dictates potential drug targets that act through allosteric modulation.[1]
When mGlu5 is in the active conformation, signaling begins with glutamate binding to the Venus flytrap domain. The signal is transduced across the cysteine-rich domain to the TMD.[3] Next, the dimerization of the TMD occurs. This activates the Gq/11 pathway, which activates phospholipase Cβ[3]. The active phospholipase Cβ hydrolyzes phosphotinositides and generates inositol 1,4,5-trisphosphate and diacyl-glycerol.[4] This results in calcium mobilization and activation of protein kinase C (PKC)[3]. Calcium is a neurotransmitter, and relatively low concentrations of calcium can cause a large response across the neuronal synapse[3]. In addition to calcium stimulating an excitory response in nerve cells, PKC can be activated for regulatory purposes by the influx of calcium. A serine on PKC can become phosphorylated, which leaves PKC unable to bind to G beta-gamma (Gßγ) protein complex[3]. Unbound Gßγ protein can then inhibit voltage-sensitive calcium channels to reduce calcium influx and provide feedback inhibition to the glutamate signaling pathway. [3].
Extracellular Domain
The extracellular domain of mGlu5 contains several key extracellular loops that will help modulate ligand binding. are shown here with extracellular loops (ECLs) 1, 2, and 3 highlighted in purple. Additionally in the ECL domain, a is attached to both Helix 3 and the amino acid chain between Helix 5 and the N-terminus. Helix 3 and Helix 5 are colored in teal and red respectively.The N-terminus is represented in blue. The disulfide bond is highlighted in yellow, and it is conserved in all classes of mGlu5 TMD[2]. The disulfide bond is critical in maintaining the position of ECL 2 [1]. ECLs and the helices are also factors that dictate how mavoglurant fits in the binding pocket [1]. The position of these ECLs can change the effective size of the binding pocket through loop positioning[1].
Binding Pocket

Figure 2. Mavoglurant in its binding pocket of the 7TM region of mGLu5 Class C receptor. The binding pocket's surface is clipped in black with the substrate, mavoglurant, in red. The rest of the protein is colored in green. The binding pocket is present in the 7 Helix Transmembrane Domain that would be present in the phospholipid bilayer as an integral protein. The presence of mavoglurant inhibits the function of the metabotropic glutamate receptor.
The binding pocket, not to be confused with the glutamate binding site, represents a useful source of regulatory control. The binding pocket is only accessible by a relatively narrow (7 Å) [1]. This small entrance severely restricts the access of both positive and negative allosteric regulators. One such regulator, , is a relatively small molecule that can travel through the entrance and fit between the helices of the mGlu5 receptor[1]. Mavoglurant acts as a negative allosteric modulator of the mGlu5 receptor[1]. Mavoglurant is a relatively hydrophobic molecule, which compliments the largely hydrophobic nature of the interior of mGlu5[1]. The carbamate tail and hydroxyl group of mavoglurant will also interact with several key amino acids in the binding site of mGlu5[1].
Important Amino Acids[1]:
- 747 forms a hydrogen bond network with the main chain carbonyl of Glycine 652 and the carbamate portion of mavoglurant.
- Bicyclic ring of mavoglurant surrounded by . Hydrophobic regions of the mGlu5 receptor are shown in purple and the rest of the helices are shown in grey. Mavoglurant is represented by spheres.
- 2 Catalytic residues H-bond to the hydroxyl oxygen of our ligand.
- A inside of the binding pocket helps stabilize the inactive state.
Upon mavoglurant’s diffusion into the mGlu5 receptor binding pocket, the ordered water cage found in the center of mGlu5 is displaced[1]. Favorable interactions between the hydrophobic regions of the binding pocket and the bicyclic ring and the 3-methylphenyl ring of mavoglurant help increase the strength and energetic favorability of mavoglurant binding via the hydrophobic effect. In the binding pocket mavoglurant can hydrogen bond with Asp 747 and that hydrogen bond will be further stabilized by an extended hydrogen bond network with Gly 652[1]. The hydroxyl group of mavoglurant will also form another hydrogen bond with Ser 805 and Ser 809[1]. Multiple hydrogen bonds make the binding of mavoglurant to the mGlu5 receptor favorable and specific[2].
Once bound to mavoglurant, transmembrane helix 7 undergoes a conformational change[1]. The shifting of TM7 will lead to a more global conformational change, which inactivates the receptor by moving intracellular loops inward [1]. This will leave the G-protien unable to bind with mGlu5[2]. Variation can be seen in positioning of alpha helices 5 and 7 across receptor class. Class C receptors have less space for mavoglurant to enter as compared to Class A and F receptors[2]. Increased specificity and stronger binding affinity could be a result of the more narrow structure of mGlu5.
Ionic Locks
Another important structural feature is the series of on the intracellular side of the protein. Interactions between these five amino acids will form a salt bridge, which will stabilize the inactive conformation[1]. The primary ionic lock forms between Glu770, Lys665, and Ser613[1]. A secondary ionic lock occurs between Ser614 and Arg668[1]. The purpose of these ionic locks is analogous to the ionic interactions that stabilize the T state in Hemoglobin. In the case of the TMD of mGlu5, the ionic lock is formed when the NAM mavoglurant is bound. These stabilize the inactive state, where the intracellular loops are stabilized facing inwards[2]. This conformational change will effectively block the crevice that is involved in binding the G-protein[2]. Models have suggested that, even in a glutamate bound state, the mavoglurant bound receptor would be dimerized but incapable of signaling[2]. This signaling incapable mGlu5 dimer will help maintain the readiness of the pathway, while still decreasing signal response.
Clinical Relevance
Role in Diseases
mGlu5 is located mainly post-synaptically and is in high abundance in the nucleus accumbens, caudate nucleus, striatum, hippocampus and cerebellar cortex.[5] These areas of the brain are highly involved in cognition, motivation, and emotion, which are essential neural functions. Diseases and other mental deficiencies arise from either an overactivation of the GPCR, which overactivates its coupled signaling pathway, or from underactivation of both the GPCR and its coupled pathway.[6] Negative allosteric modulators (NAMs) work to decrease protein activity and are being studied as treatments for fragile X-syndrome, depression, anxiety and dyskinesia.[1] Conversely, positive allosteric modulators (PAMs) work to increase protein activity and are being studied for the treatment of schizophrenia and cognitive disorders.[6]
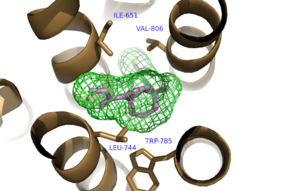
Figure 3. Hydrophobic Pocket Surrounding Mavoglurant
Interactions with a Negative Allosteric Modulator
The structure of mGlu5 bound to the NAM Mavoglurant demonstrates how protein activity is decreased through drug interactions.
binds within the allosteric binding site in the core of the seven trans-membrane α-helices, having passed through the restricted entrance formed by the . Bound Mavoglurant forms multiple interactions with the protein that further stabilize the inactive conformation. The bicyclic ring system of the drug is surrounded by a pocket of mainly hydrophobic residues including Val 806, Met 802, Phe 788, Trp 785, Leu 744, Ile 651, Pro 655, and Asn 747 (Figure 3).[1] The carbamate tail of Mavoglurant forms a hydrogen bond through its carbonyl oxygen to the amide side-chain of Asn 747 of TM4. A hydroxyl group similarly forms hydrogen bonds to mGlu5, specifically at two serine residues (S805 and S809) of TM7. These residues form a hydrogen bonding network to other residues through their main chain atoms and a coordinated water molecule. The interactions between Mavoglurant and mGlu5 involve TM helices that were not previously stabilized by any strong interactions, introducing a new level of stability that favors the inactive conformation of the protein and hence decreases the overall activity of mGlu5.[1]
Fragile X
Fragile X syndrome is the most common genetic cause of mental disability, and is a member of the Autism spectrum disorder family.[7] The severity of intellectual disability can vary from patient to patient, but symptoms commonly stem from a misregulation of the mGlu1 and MGlu5 pathways[7]. Misregulation of these pathways leads to over potentiation in neural cells. and other allosteric regulators like fenobam have shown promise in treating Fragile X. One positive characteristic of ligands that target the TMD of mGlu5 is they tend to be more specific, thus interacting less with nonspecific brain proteins.[8] Mavoglurant down regulates glutamate signaling in an attempt to decrease potentiation. Unfortunately, recent Phase 2 clinical trials have proven mavoglurant ineffective [7]. However, modulators of mGlu5 TMD are still being investigated as treatment for Parkinson's, Alzheimer's disease, and various addictions[3].
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, Errey JC, Jazayeri A, Khan S, Tehan B, Weir M, Wiggin GR, Marshall FH. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014 Jul 31;511(7511):557-62. doi: 10.1038/nature13396. Epub 2014 Jul 6. PMID:25042998 doi:http://dx.doi.org/10.1038/nature13396
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y, Niswender CM, Katritch V, Meiler J, Cherezov V, Conn PJ, Stevens RC. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science. 2014 Apr 4;344(6179):58-64. doi: 10.1126/science.1249489. Epub 2014 Mar , 6. PMID:24603153 doi:http://dx.doi.org/10.1126/science.1249489
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295-322. doi:, 10.1146/annurev.pharmtox.011008.145533. PMID:20055706 doi:http://dx.doi.org/10.1146/annurev.pharmtox.011008.145533
- ↑ Woodcock EA, Kistler PM, Ju YK. Phosphoinositide signalling and cardiac arrhythmias. Cardiovasc Res. 2009 May 1;82(2):286-95. doi: 10.1093/cvr/cvn283. Epub 2008 Oct, 20. PMID:18940816 doi:http://dx.doi.org/10.1093/cvr/cvn283
- ↑ Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993 Nov 26;163(1):53-7. PMID:8295733
- ↑ 6.0 6.1 Li G, Jorgensen M, Campbell BM. Metabotropic glutamate receptor 5-negative allosteric modulators for the treatment of psychiatric and neurological disorders (2009-July 2013). Pharm Pat Anal. 2013 Nov;2(6):767-802. doi: 10.4155/ppa.13.58. PMID:24237242 doi:http://dx.doi.org/10.4155/ppa.13.58
- ↑ 7.0 7.1 7.2 Bailey DB Jr, Berry-Kravis E, Wheeler A, Raspa M, Merrien F, Ricart J, Koumaras B, Rosenkranz G, Tomlinson M, von Raison F, Apostol G. Mavoglurant in adolescents with fragile X syndrome: analysis of Clinical Global Impression-Improvement source data from a double-blind therapeutic study followed by an open-label, long-term extension study. J Neurodev Disord. 2016;8:1. doi: 10.1186/s11689-015-9134-5. Epub 2015 Dec 15. PMID:26855682 doi:http://dx.doi.org/10.1186/s11689-015-9134-5
- ↑ Feng Z, Ma S, Hu G, Xie XQ. Allosteric Binding Site and Activation Mechanism of Class C G-Protein Coupled Receptors: Metabotropic Glutamate Receptor Family. AAPS J. 2015 May;17(3):737-53. doi: 10.1208/s12248-015-9742-8. Epub 2015 Mar 12. PMID:25762450 doi:http://dx.doi.org/10.1208/s12248-015-9742-8
External Resources
Novartis Fragile X trials
Mavoglurant as a treatment for Parkinson's disease
Pharmacology of mGlu5 receptors
Glutamate signaling pathway and receptors general overview
Gq Alpha subunit



