User:R. Jeremy Johnson/Lysophosphatidic acid receptor 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Lysophosphatidic Acid Receptor 1== | ==Lysophosphatidic Acid Receptor 1== | ||
| - | <StructureSection load='4z34' size='340' side='right' caption='Cartoon representation of the LPA1 protein and its antagonist, ON7, colored in white. ([https://en.wikipedia.org/wiki/Protein_Data_Bank PDB] code [http://www.rcsb.org/pdb/explore/explore.do?structureId=4z34 4Z34]) ' scene='72/ | + | <StructureSection load='4z34' size='340' side='right' caption='Cartoon representation of the LPA1 protein and its antagonist, ON7, colored in white. ([https://en.wikipedia.org/wiki/Protein_Data_Bank PDB] code [http://www.rcsb.org/pdb/explore/explore.do?structureId=4z34 4Z34]) ' scene='72/721545/Overall/2'> |
| - | + | ||
== Introduction == | == Introduction == | ||
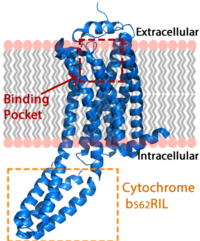
| - | Lysophosphatidic Acid Receptor 1 (commonly referred to as LPA<sub>1</sub>) is a [[G protein-coupled receptor]] and one of 6 different LPA receptors (LPA<sub>1</sub>-LPA<sub>6</sub>). These receptors bind the phospholipid derivative [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a signaling molecule that acts as a potent [https://en.wikipedia.org/wiki/Mitogen mitogen] upon binding to one of its six receptors.<ref name="regpeps">PMID: 26091040</ref> LPA<sub>1</sub> is part of the larger [http://jb.oxfordjournals.org/content/131/6/767 EDG receptor family], which includes the more widely studied sphingosine 1-phopshate receptors.<ref name="regpeps">PMID: 26091040</ref> This receptor is responsible for initiating several different signaling cascades with different molecules and G-proteins.<ref name = 'Yung'>Yung, Y. C., N. C. Stoddard, and J. Chun. "LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology." The Journal of Lipid Research 55.7 (2014): 1192-214. Web. 17 Feb. 2016.' </ref> These cascades ultimately result in growth, survival, and movement of cells, as well as neural cell development.<ref name = 'Chun'>Chun, J., Hla, T., Spiegel, S., and Moolenaar, W.H. “Lysophospholipid Receptors: Signaling and Biochemistry.” John Wiley & Sons, Inc. (2013) pp.i-xviii. 5 Feb. 2016.' </ref> | + | Lysophosphatidic Acid Receptor 1 (commonly referred to as LPA<sub>1</sub>) is a [[G protein-coupled receptor]] and one of 6 different LPA receptors (LPA<sub>1</sub>-LPA<sub>6</sub>). These receptors bind the phospholipid derivative [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a signaling molecule that acts as a potent [https://en.wikipedia.org/wiki/Mitogen mitogen] upon binding to one of its six receptors.<ref name="regpeps">PMID: 26091040</ref> LPA<sub>1</sub> is part of the larger [http://jb.oxfordjournals.org/content/131/6/767 EDG receptor family], which includes the more widely studied sphingosine 1-phopshate receptors.<ref name="regpeps">PMID: 26091040</ref> This receptor is responsible for initiating several different signaling cascades with different molecules and G-proteins.<ref name = 'Yung'>Yung, Y. C., N. C. Stoddard, and J. Chun. "LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology." The Journal of Lipid Research 55.7 (2014): 1192-214. Web. 17 Feb. 2016.' </ref> These cascades ultimately result in growth, survival, and movement of cells, as well as neural cell development.<ref name = 'Chun'>Chun, J., Hla, T., Spiegel, S., and Moolenaar, W.H. “Lysophospholipid Receptors: Signaling and Biochemistry.” John Wiley & Sons, Inc. (2013) pp.i-xviii. 5 Feb. 2016.' </ref> 72/721543/Overallrainbow2/1 [[Image:LPA_in_membrane4.fw.png|200px|center|thumb|'''Figure 1:''' LPA receptor (blue) bound to the cell membrane. The binding pocket is highlighted in red. The added bRIL protein is highlighted in orange.]] |
== Lysophosphatidic Acid == | == Lysophosphatidic Acid == | ||
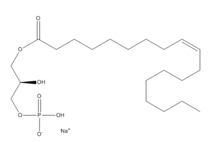
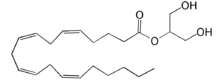
| - | + | [[Image:LPA.png|220px|left|thumb|'''Figure 2:''' Chemical Structure of LPA (monoacyl-sn-glycero-3-phosphate)]] Lysophosphatidic Acid (LPA) consists of an unsaturated fatty acid chain, a glycerol backbone, and a free phosphate group (Figure 2). Lysophosphatidic acid is found in nearly all cells, tissues, and fluids of the body.<ref name= "Chrencik"> DOI: 10.1016/j.cell.2015.06.002 </ref> LPA is present intracellularly as a precursor of phospholipid biosynthesis, and extracellularly as a signalling phospholipid. | |
| - | [[Image:LPA.png|220px|left|thumb|'''Figure 2:''' Chemical Structure of LPA (monoacyl-sn-glycero-3-phosphate)]] | + | |
| - | Lysophosphatidic Acid (LPA) consists of an unsaturated fatty acid chain, a glycerol backbone, and a free phosphate group (Figure 2). Lysophosphatidic acid is found in nearly all cells, tissues, and fluids of the body.<ref name= "Chrencik"> DOI: 10.1016/j.cell.2015.06.002 </ref> LPA is present intracellularly as a precursor of phospholipid biosynthesis, and extracellularly as a signalling phospholipid. | + | |
Extracellularly, LPA is produced from lysophosphatidylcholine by the enzyme autotaxin.<ref name= "Chrencik"/> Autotaxin was originally linked with metastasis, and this link was later discovered to be mediated through the production of LPA, which signals cell proliferation.<ref name= "Boutin"> DOI: 10.1007/s00018-009-0056-9 </ref> All of LPA’s activities are receptor mediated; the signalling lipid interacts with at least six G-protein coupled receptors LPA<sub>1</sub>-LPA<sub>6</sub>. | Extracellularly, LPA is produced from lysophosphatidylcholine by the enzyme autotaxin.<ref name= "Chrencik"/> Autotaxin was originally linked with metastasis, and this link was later discovered to be mediated through the production of LPA, which signals cell proliferation.<ref name= "Boutin"> DOI: 10.1007/s00018-009-0056-9 </ref> All of LPA’s activities are receptor mediated; the signalling lipid interacts with at least six G-protein coupled receptors LPA<sub>1</sub>-LPA<sub>6</sub>. | ||
| Line 19: | Line 16: | ||
=== Structural Stabilization === | === Structural Stabilization === | ||
| - | |||
Three native <scene name='72/721545/Disulfides/5'>disulfide bonds</scene> in the extracellular region of this receptor provide fold stability.<ref name= "Chrencik"/> The first disulfide bond constrains the N terminal helix to extracellular loop(ECL) 2. The second disulfide bond shapes ECL2, and the third binds ECL3 to one of the transmembrane alpha helices. These disulfide bonds provide intramolecular stabilization along the extracellular region of the LPA<sub>1</sub> receptor, where the substrate enters into the binding pocket. The <scene name='72/721545/N-terminus/3'>N-terminus</scene> is a six turn alpha helix. It functions like a cap on the extracellular side of the protein, packing tightly against ECL1 and ECL2.<ref name= "Chrencik"/> The N-terminus helix also provides <scene name='72/721545/34_39_40/4'>polar amino acids</scene> that interact with the ligand when bound. The extracellular region of this receptor plays a role in substrate specificity. | Three native <scene name='72/721545/Disulfides/5'>disulfide bonds</scene> in the extracellular region of this receptor provide fold stability.<ref name= "Chrencik"/> The first disulfide bond constrains the N terminal helix to extracellular loop(ECL) 2. The second disulfide bond shapes ECL2, and the third binds ECL3 to one of the transmembrane alpha helices. These disulfide bonds provide intramolecular stabilization along the extracellular region of the LPA<sub>1</sub> receptor, where the substrate enters into the binding pocket. The <scene name='72/721545/N-terminus/3'>N-terminus</scene> is a six turn alpha helix. It functions like a cap on the extracellular side of the protein, packing tightly against ECL1 and ECL2.<ref name= "Chrencik"/> The N-terminus helix also provides <scene name='72/721545/34_39_40/4'>polar amino acids</scene> that interact with the ligand when bound. The extracellular region of this receptor plays a role in substrate specificity. | ||
=== Key Ligand Interactions === | === Key Ligand Interactions === | ||
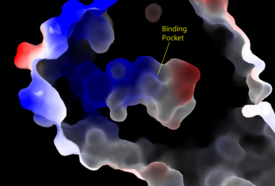
| - | [[Image:Amphbindingfinal.png|275 px|right|thumb|'''Figure 3''': Electrostatic illustration of the amphipathic binding pocket of the LPA<sub>1</sub> receptor. This binding pocket was revealed by cutting away the exterior or the protein. This binding pocket, located in the interior of the protein, has both polar and nonpolar regions. The blue and red coloration highlight the positively and negatively charged regions, respectively, and the white color shows the nonpolar region of the binding pocket.]] | + | [[Image:Amphbindingfinal.png|275 px|right|thumb|'''Figure 3''': Electrostatic illustration of the amphipathic binding pocket of the LPA<sub>1</sub> receptor. This binding pocket was revealed by cutting away the exterior or the protein. This binding pocket, located in the interior of the protein, has both polar and nonpolar regions. The blue and red coloration highlight the positively and negatively charged regions, respectively, and the white color shows the nonpolar region of the binding pocket.]] The biological ligand of the LPA<sub>1</sub> receptor receptor is [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a phospholipid that contains a long, nonpolar tail, a phosphate head, a chiral hydroxyl group, and an ester group. This receptor provides specificity for its ligand by the amphipathic binding pocket; the positive region on the left hand side of the pocket stabilizes the LPA's phosphate group, the nonpolar region at the bottom of the binding pocket stabilizes the hydrophobic tail of LPA, and the polar region at the top of the pocket stabilize binding of the ester and hydroxyl group (Figure 3). The <scene name='72/721545/Ligand/4'>binding pocket</scene> for LPA consists of both polar and nonpolar residues. <scene name='72/721545/All_polar_interactions/7'>Polar</scene> residues are located on the N terminus and within the binding pocket. A <scene name='72/721545/Hydrophobic_pocket/4'>hydrophobic pocket</scene> also interacts with the long acyl chain of LPA. The shape and polarity of the binding pocket makes it specific for molecules with a polar head and long hydrophobic tail shaped like LPA. |
| - | The biological ligand of the LPA<sub>1</sub> receptor receptor is [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a phospholipid that contains a long, nonpolar tail, a phosphate head, a chiral hydroxyl group, and an ester group. This receptor provides specificity for its ligand by the amphipathic binding pocket; the positive region on the left hand side of the pocket stabilizes the LPA's phosphate group, the nonpolar region at the bottom of the binding pocket stabilizes the hydrophobic tail of LPA, and the polar region at the top of the pocket stabilize binding of the ester and hydroxyl group (Figure 3). The <scene name='72/721545/Ligand/4'>binding pocket</scene> for LPA consists of both polar and nonpolar residues. <scene name='72/721545/All_polar_interactions/7'>Polar</scene> residues are located on the N terminus and within the binding pocket. A <scene name='72/721545/Hydrophobic_pocket/4'>hydrophobic pocket</scene> also interacts with the long acyl chain of LPA. The shape and polarity of the binding pocket makes it specific for molecules with a polar head and long hydrophobic tail shaped like LPA. | + | |
[http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8589 ONO-9780307 (ON7)] is an antagonist for LPA due to its large nonpolar region, chiral hydroxyl group, ester, and carboxylic acid which all resemble portions of the LPA molecule (Figure 3). Four separate interactions with this antagonist of LPA<sub>1</sub> help demonstrate the key interactions that stabilize the binding of the LPA phospholipid to this receptor. In the nonpolar region of the binding pocket, <scene name='72/721543/Nonpolar/2'>three non polar residues</scene> of LPA<sub>1</sub> stabilize the large nonpolar group of ON7. At the polar region, the ligand binding is stabilized by <scene name='72/721543/Arg124gln125/4'>Arg124 and Glu125</scene> forming ionic and polar interactions with the carboxylic acid and the hydroxyl group of ON7.<ref name="regpeps">PMID: 26091040</ref> In addition, interplay between <scene name='72/721543/Lys39_and_glu293/8'>Glu293 and Lys39</scene> causes another stabilizing component with the ON7 antagonist. Glu293 forms polar interactions with Lys39, positioning it in close proximity to to the carboxylic acid of ON7, which then interactions with Lys39 via ionic bonding.<ref name="regpeps">PMID: 26091040</ref> While Lys39 is highly conserved among all six LPA receptors, a neighboring histidine residue is specific to the LPA<sub>1</sub> receptor. <scene name='72/721543/His40/4'>His40</scene> forms both ionic and polar interactions with the carboxylic acid of ON7. Protonation of this residue greatly affects the binding affinity of LPA, leading to an increase in the pathways associated with cell proliferation and migration. Because cancerous tumors create acidic environments where His40 is protonated, this residue is an important link to tumor growth and cancer cell movement.<ref name="regpeps">PMID: 26091040</ref> | [http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8589 ONO-9780307 (ON7)] is an antagonist for LPA due to its large nonpolar region, chiral hydroxyl group, ester, and carboxylic acid which all resemble portions of the LPA molecule (Figure 3). Four separate interactions with this antagonist of LPA<sub>1</sub> help demonstrate the key interactions that stabilize the binding of the LPA phospholipid to this receptor. In the nonpolar region of the binding pocket, <scene name='72/721543/Nonpolar/2'>three non polar residues</scene> of LPA<sub>1</sub> stabilize the large nonpolar group of ON7. At the polar region, the ligand binding is stabilized by <scene name='72/721543/Arg124gln125/4'>Arg124 and Glu125</scene> forming ionic and polar interactions with the carboxylic acid and the hydroxyl group of ON7.<ref name="regpeps">PMID: 26091040</ref> In addition, interplay between <scene name='72/721543/Lys39_and_glu293/8'>Glu293 and Lys39</scene> causes another stabilizing component with the ON7 antagonist. Glu293 forms polar interactions with Lys39, positioning it in close proximity to to the carboxylic acid of ON7, which then interactions with Lys39 via ionic bonding.<ref name="regpeps">PMID: 26091040</ref> While Lys39 is highly conserved among all six LPA receptors, a neighboring histidine residue is specific to the LPA<sub>1</sub> receptor. <scene name='72/721543/His40/4'>His40</scene> forms both ionic and polar interactions with the carboxylic acid of ON7. Protonation of this residue greatly affects the binding affinity of LPA, leading to an increase in the pathways associated with cell proliferation and migration. Because cancerous tumors create acidic environments where His40 is protonated, this residue is an important link to tumor growth and cancer cell movement.<ref name="regpeps">PMID: 26091040</ref> | ||
| - | |||
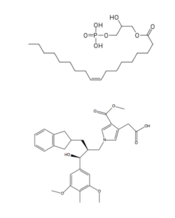
[[Image:Compare.png|175 px|left|thumb|'''Figure 4''': Structures of LPA and its antagonist, ON7.]] | [[Image:Compare.png|175 px|left|thumb|'''Figure 4''': Structures of LPA and its antagonist, ON7.]] | ||
| - | |||
== Function == | == Function == | ||
The LPA<sub>1</sub> receptor is present in nearly all cells and tissues, and deletion of the LPA<sub>1</sub> receptor has physiological effects on every organ system, indicating its wide range of functions. Specifically, this receptor initiates downstream signaling cascades with three G<sub>α</sub> proteins: G<sub>i/o</sub>, G<sub>q/11</sub>, and G<sub>12/13</sub>. Specifically, G<sub>α</sub> proteins begin signaling cascades that activate [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C] and [[MAPK]]s that signal for cell proliferation, survival, and migration. <ref name = 'Yung'>Yung, Y. C., N. C. Stoddard, and J. Chun. "LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology." The Journal of Lipid Research 55.7 (2014): 1192-214. Web. 17 Feb. 2016.' </ref> Despite this receptor being expressed ubiquitously, LPA<sub>1</sub> is highly expressed in neural tissue, aiding in several different neurodevelopmental processes including growth and folding of the [https://www.dartmouth.edu/~rswenson/NeuroSci/chapter_11.html cerebral cortex] and the growth, survival, and migration of neural [https://en.wikipedia.org/wiki/Progenitor_cell progenitor cells].<ref name = 'Chun'>Chun, J., Hla, T., Spiegel, S., and Moolenaar, W.H. “Lysophospholipid Receptors: Signaling and Biochemistry.” John Wiley & Sons, Inc. (2013) pp.i-xviii. 5 Feb. 2016.' </ref> Finally, LPA<sub>1</sub> receptors expressed in neural tissue act through a signaling pathway with the [https://en.wikipedia.org/wiki/RAC1 Rac1] G-protein to aid in Schwann cell migration and myelination.<ref name="number5">PMID: 24115248</ref> | The LPA<sub>1</sub> receptor is present in nearly all cells and tissues, and deletion of the LPA<sub>1</sub> receptor has physiological effects on every organ system, indicating its wide range of functions. Specifically, this receptor initiates downstream signaling cascades with three G<sub>α</sub> proteins: G<sub>i/o</sub>, G<sub>q/11</sub>, and G<sub>12/13</sub>. Specifically, G<sub>α</sub> proteins begin signaling cascades that activate [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C] and [[MAPK]]s that signal for cell proliferation, survival, and migration. <ref name = 'Yung'>Yung, Y. C., N. C. Stoddard, and J. Chun. "LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology." The Journal of Lipid Research 55.7 (2014): 1192-214. Web. 17 Feb. 2016.' </ref> Despite this receptor being expressed ubiquitously, LPA<sub>1</sub> is highly expressed in neural tissue, aiding in several different neurodevelopmental processes including growth and folding of the [https://www.dartmouth.edu/~rswenson/NeuroSci/chapter_11.html cerebral cortex] and the growth, survival, and migration of neural [https://en.wikipedia.org/wiki/Progenitor_cell progenitor cells].<ref name = 'Chun'>Chun, J., Hla, T., Spiegel, S., and Moolenaar, W.H. “Lysophospholipid Receptors: Signaling and Biochemistry.” John Wiley & Sons, Inc. (2013) pp.i-xviii. 5 Feb. 2016.' </ref> Finally, LPA<sub>1</sub> receptors expressed in neural tissue act through a signaling pathway with the [https://en.wikipedia.org/wiki/RAC1 Rac1] G-protein to aid in Schwann cell migration and myelination.<ref name="number5">PMID: 24115248</ref> | ||
| - | |||
| - | |||
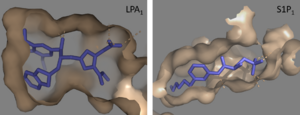
== Receptor Comparison == | == Receptor Comparison == | ||