User:R. Jeremy Johnson/Glucagon Receptor
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
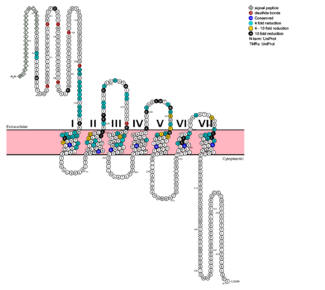
G protein coupled receptors (GPCRs) are the largest class of integral membrane proteins. GPCRs are divided into five families; the rhodopsin family (class A), the secretin family (class B), the glutamate family (class C), the frizzled/taste family (class F), and the adhesion family.<ref name= "Zhang 2006"/> Roughly 5% of the human genome encodes g protein-coupled receptors, which are responsible for the transduction of endogenous signals and the instigation of cellular responses.<ref name= "Zhang 2006"/> All GPCRs contain a similar seven α-helical transmembrane domain <scene name='72/727091/Full_Structure_with_Labels/1'>(TMD or 7TMD)</scene> that once bound to its ligand, undergoes a conformational change and tranduces a signal to coupled, heterotrimeric G proteins. The initiation of intracellular signal pathways occur in response to stimuli such as light, Ca2+, amino acids, nucleotides, odorants, peptides, and other proteins, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor#Physiological_roles and accomplishes many interesting physiological roles]. <ref name= "Zhang 2006">DOI 10.1371/journal.pcbi.0020013</ref> The '''human glucagon receptor''' ('''GCGR''') is one of 15 secretin-like, or Class B, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptors] (GPCRs). Like other GPCRs, it has a <scene name='72/721538/7tm_labeled_helicies/3'>7 trans-membrane </scene> helical domain and a globular N-terminus <scene name='72/721538/Ecd/2'>extracellular domain</scene> (ECD). [[Image:Protter GLR HUMAN.png |325 px|left|thumb|'''Figure 1''': Snake Plot of GCGR TMD. Residues of particular importance in glucagon binding affinity are found in green, yellow, and black. Residues in red are the location of critical disulfide bonds, while blue residues were found to be highly conserved across all class B GPCRs.<ref name= "Siu 2013"/>]] | G protein coupled receptors (GPCRs) are the largest class of integral membrane proteins. GPCRs are divided into five families; the rhodopsin family (class A), the secretin family (class B), the glutamate family (class C), the frizzled/taste family (class F), and the adhesion family.<ref name= "Zhang 2006"/> Roughly 5% of the human genome encodes g protein-coupled receptors, which are responsible for the transduction of endogenous signals and the instigation of cellular responses.<ref name= "Zhang 2006"/> All GPCRs contain a similar seven α-helical transmembrane domain <scene name='72/727091/Full_Structure_with_Labels/1'>(TMD or 7TMD)</scene> that once bound to its ligand, undergoes a conformational change and tranduces a signal to coupled, heterotrimeric G proteins. The initiation of intracellular signal pathways occur in response to stimuli such as light, Ca2+, amino acids, nucleotides, odorants, peptides, and other proteins, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor#Physiological_roles and accomplishes many interesting physiological roles]. <ref name= "Zhang 2006">DOI 10.1371/journal.pcbi.0020013</ref> The '''human glucagon receptor''' ('''GCGR''') is one of 15 secretin-like, or Class B, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptors] (GPCRs). Like other GPCRs, it has a <scene name='72/721538/7tm_labeled_helicies/3'>7 trans-membrane </scene> helical domain and a globular N-terminus <scene name='72/721538/Ecd/2'>extracellular domain</scene> (ECD). [[Image:Protter GLR HUMAN.png |325 px|left|thumb|'''Figure 1''': Snake Plot of GCGR TMD. Residues of particular importance in glucagon binding affinity are found in green, yellow, and black. Residues in red are the location of critical disulfide bonds, while blue residues were found to be highly conserved across all class B GPCRs.<ref name= "Siu 2013"/>]] | ||
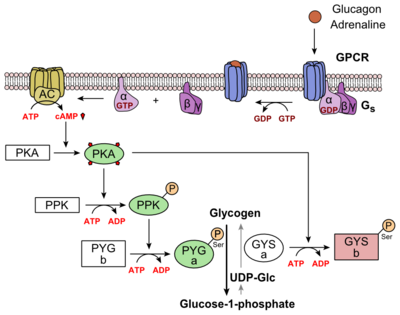
| - | Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases the intracellular concentration of cAMP, inositol phosphate, and calcium levels. <ref>DOI 10.1111/bph.12689</ref> These secondary messengers are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest as disease targets. <ref name= "Hollenstein 2014">DOI 10.1016/j.tips.2013.11.001</ref> Structural approaches to the development of agonists and antagonists have however been hampered by the lack of accurate Class B TMD visualizations. Recent crystal structure images of corticoptropin-releasing factor receptor 1 (PDB: 4K5Y) and human glucagon receptor (PDB: 4L6R) were accomplished through x-ray crystallography. <ref name= "Hollenstein 2013">DOI 10.1038/nature12357</ref> <ref name= "Siu 2013">DOI 10.1038/nature12393</ref> | + | Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases the intracellular concentration of cAMP, inositol phosphate, and calcium levels. <ref>DOI 10.1111/bph.12689</ref> These secondary messengers are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest as disease targets. <ref name= "Hollenstein 2014">DOI 10.1016/j.tips.2013.11.001</ref> Structural approaches to the development of agonists and antagonists have however been hampered by the lack of accurate Class B TMD visualizations. Recent crystal structure images of corticoptropin-releasing factor receptor 1 (PDB: 4K5Y) and human glucagon receptor (PDB: 4L6R) were accomplished through x-ray crystallography. <ref name= "Hollenstein 2013">DOI 10.1038/nature12357</ref><ref name= "Siu 2013">DOI 10.1038/nature12393</ref> |
==Structures of Class A vs. Class B GPCRs== | ==Structures of Class A vs. Class B GPCRs== | ||
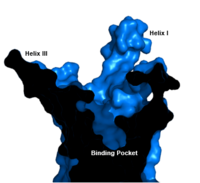
| - | Class A vs. class B glucagon receptors share less than fifteen percent sequence homology, but both share a 7TM domain. <ref name=" | + | Class A vs. class B glucagon receptors share less than fifteen percent sequence homology, but both share a 7TM domain.<ref name= "Hollenstein 2013"/> Understanding for class A family of GCGRs of the structure-function [https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme_Catalytic_Mechanism mechanism] has made great progress over the past few years, but understanding of class B has fallen behind but is now catching up.<ref name= "Siu 2013"/> Comparison of the <scene name='72/721536/Final_1st_image/1'>class B 7TM</scene> helices to that of the <scene name='72/721536/Final_class_a_7tm/1'>class A 7TM</scene> helices showed that the general orientation and positioning of the [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] are conserved through both classes. Detailed structural alignments of the two GPCR subclasses revealed multiple sequence misalignments in the transmembrane region signifying a variety of structural deviations in the transmembrane helices.<ref name= "Siu 2013"/> The N-terminal end of <scene name='72/721536/Final_helix_1/2'>helix one</scene> in class B GCGR, located in the 7TM, is longer than any known class A GPCR structure and stretches three supplementary helical turns above the extracellular (EC) membrane boundary. This region is referred to as the <scene name='72/721535/stalk/1'>stalk</scene>. The stalk is involved in glucagon binding and helps in defining the orientation of the ECD with respect to the 7TM domain.<ref name= "Siu 2013"/> Also specific to class B GPCRs, a [https://en.wikipedia.org/wiki/Glycine Gly] residue at position 393 induces a <scene name='72/721535/Helical_bend/4'>bend in helix VII</scene>; this bend is stabilized by the [http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Hydrophobic_Interactions hydrophobic interaction] between the <scene name='72/721535/Gly_393_phe_184/2'> glycine 393 and phenylalanine 184</scene>. One of the most distinguishable characteristics of the class B 7TM is the <scene name='72/721536/Class_b_helix_8_tilt_finals/1'>helix VIII tilt</scene> of 25 degrees and its length compared to that of <scene name='72/721536/Class_a_helix_vii_tilt/2'>class A helix VIII tilt</scene>, which is much shorter. This helical tilt results from [https://en.wikipedia.org/wiki/Phenylalanine Glu] 406 in helix VIII that is fully conserved in secretin-like receptors and forms two interhelical <scene name='72/721537/Salt_bridges/2'>salt bridges</scene> with [https://simple.wikipedia.org/wiki/Conserved_sequence conserved residues] [https://en.wikipedia.org/wiki/Arginine Arg] 173 and Arg 346.<ref name= "Siu 2013"/> Despite these differences, a vital region that is conserved in both class B and class A receptors is the [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin_actual/2'>Cys 294 and Cys 224</scene> in extracellular loop two (ECL2). This bond stabilizes the receptors entire 7TM fold. Lastly, the locations of the extracellular tips for class B glucagon receptors allow for a much wider and deeper [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand-binding pocket] than any of the class A GPCRs.<ref name= "Siu 2013"/> While interface interactions between helices VI, V, and III, are not unique to class B receptors because certain homologous and even conserved residues exist in class A receptors (like [https://en.wikipedia.org/wiki/Tyrosine Tyr] 239 and Leu 358), as a part of the interface <scene name='72/721537/Helical_interactions_vi-v-iii/2'>stabilization</scene> between helices VI, V, and III, a Class B-specific [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond] occurs between [https://en.wikipedia.org/wiki/Asparagine Asn] 318 of Helix V and [https://en.wikipedia.org/wiki/Leucine Leu] 242 of Helix III.<ref name= "Siu 2013"/> |
===How These Structures Lead to Function=== | ===How These Structures Lead to Function=== | ||
[[Image:Closed and open conformation.png|(|):|250 px|left|thumb|'''Figure 2: Open conformation in contrast to the closed conformation.''' The movement of the single helix over the top of the transmembrane domain is the most distinguishable characteristic between closed and open conformation. The <scene name='72/721535/Stalk/1'>stalk</scene> is not accessible to glucagon in the closed conformation.<ref name="Lastt">PMID: 26227798</ref>]] | [[Image:Closed and open conformation.png|(|):|250 px|left|thumb|'''Figure 2: Open conformation in contrast to the closed conformation.''' The movement of the single helix over the top of the transmembrane domain is the most distinguishable characteristic between closed and open conformation. The <scene name='72/721535/Stalk/1'>stalk</scene> is not accessible to glucagon in the closed conformation.<ref name="Lastt">PMID: 26227798</ref>]] | ||
| - | Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels. <ref name=" | + | Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels.<ref name= "Siu 2013"/> The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide [https://en.wikipedia.org/wiki/Receptor_(biochemistry) receptors]. <ref name="Ligands">PMID: 21542831</ref> The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed [https://en.wikipedia.org/wiki/Conformation conformation] of the GCGR (Figure 2). The open conformation is when glucagon can bind to GCGR; in the closed conformation binding does not occur.<ref name="Ligands">PMID: 21542831</ref> |
==Glucagon Receptor (GCGR)== | ==Glucagon Receptor (GCGR)== | ||
| Line 21: | Line 21: | ||
=== GCGR-Specific Traits === | === GCGR-Specific Traits === | ||
==== Helix I Stalk Region ==== | ==== Helix I Stalk Region ==== | ||
| - | The tip of Helix I extends above the cell membrane into the extracellular space creating a <scene name='72/721538/Helix_i/14'> stalk region</scene>. This region is longer than any other class of GPCR and extends three α-helical turns above the plane of the membrane. It is proposed that the stalk helps to capture the glucagon peptide and facilitates it's insertion into the 7tm<ref name = | + | The tip of Helix I extends above the cell membrane into the extracellular space creating a <scene name='72/721538/Helix_i/14'> stalk region</scene>. This region is longer than any other class of GPCR and extends three α-helical turns above the plane of the membrane. It is proposed that the stalk helps to capture the glucagon peptide and facilitates it's insertion into the 7tm.<ref name= "Siu 2013"/> |
==== Intracellular Helix VIII ==== | ==== Intracellular Helix VIII ==== | ||
| Line 33: | Line 33: | ||
===Glucagon Binding=== | ===Glucagon Binding=== | ||
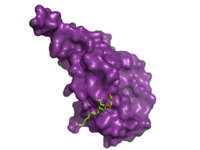
| - | The large, soluble N-terminal extracellular domains (ECD) of GCGR provide initial ligand selectivity with the deep, ligand pocket of the TMD providing secondary recognition.<ref name= "Yang 2015"/> [[Image:ECD_bound_to_glucagon.png|200 px|left|thumb|'''Figure 4. Bound Molecule of Glucagon.''' A molecule of glucagon is shown bound to the GCGR's ECD (shown in magenta)]] The active, or open conformation, is characterized by an intracellular outward movement of <scene name='72/721538/Helix_v_and_vi/1'>helicies V and VI</scene> (breaking hydrogen bonds between <scene name='72/721538/Arg173-ser350_h_bond/1'>Arg173-Ser350</scene> and <scene name='72/721538/Arg173-ser350_h_bond/2'>Glu245-Thr351</scene>)<ref name= | + | The large, soluble N-terminal extracellular domains (ECD) of GCGR provide initial ligand selectivity with the deep, ligand pocket of the TMD providing secondary recognition.<ref name= "Yang 2015"/> [[Image:ECD_bound_to_glucagon.png|200 px|left|thumb|'''Figure 4. Bound Molecule of Glucagon.''' A molecule of glucagon is shown bound to the GCGR's ECD (shown in magenta)]] The active, or open conformation, is characterized by an intracellular outward movement of <scene name='72/721538/Helix_v_and_vi/1'>helicies V and VI</scene> (breaking hydrogen bonds between <scene name='72/721538/Arg173-ser350_h_bond/1'>Arg173-Ser350</scene> and <scene name='72/721538/Arg173-ser350_h_bond/2'>Glu245-Thr351</scene>)<ref name= "Hollenstein 2014"/> and an extracellular rotation of the ECD until it is almost perpendicular to the membrane surface.<ref name="Lastt"/> While the stalk region of Helix I helps to facilitate the motion of the ECD, intracellular G-protein coupling and extracellular glucagon binding stabilized this active state. In the abscence of glucagon, however, the GCGR adopts a closed conformation in which all three of the extracellular loops of the 7tm (<scene name='72/721538/Ecls/1'>ECL1, ECL2, and ECL3</scene>) can interact with the ECD.<ref name="Lastt"/> In this closed state, the ECD covers the extracellular surface of the 7tm. To transition between states, the ECD rotates and moves down towards the 7tm domain. This transition mechanism is consistent with the "two-domain" binding mechanism of class B GCPRs in which (1) the C-terminus of the ligand first binds to the ECD allowing (2) the N-terminus of the ligand to interact with the 7tm and activate the protein.<ref name= "Hollenstein 2014"/> |
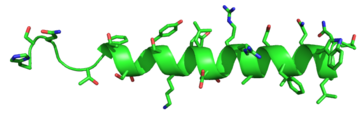
The [https://en.wikipedia.org/wiki/Residue_(chemistry) residues] in the binding pocket that are in direct contact with the glucagon molecule are [https://en.wikipedia.org/wiki/Chemical_polarity polar] (utilizing the attraction of opposite charges or [https://en.wikipedia.org/wiki/Dipole dipoles] for glucagon binding) or are hydrophobic (utilizing the hydrophobic effect). The [https://en.wikipedia.org/wiki/Active_site binding site] location of the hormone peptide ligand has been identified, and the N-terminus of glucagon is known to bind partly with the ECD while the rest of glucagon binds deep into the binding pocket. The [https://en.wikipedia.org/wiki/Amino_acid amino acids] at the N-terminus of the class B 7TM have the ability to form [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds] and [https://en.wikipedia.org/wiki/Ionic_bonding ionic interactions], which can be seen in the [https://en.wikipedia.org/wiki/Peptide_sequence amino acid sequence] of glucagon (Figure 5). <ref name="Sequence">PMID: 11946536</ref> | The [https://en.wikipedia.org/wiki/Residue_(chemistry) residues] in the binding pocket that are in direct contact with the glucagon molecule are [https://en.wikipedia.org/wiki/Chemical_polarity polar] (utilizing the attraction of opposite charges or [https://en.wikipedia.org/wiki/Dipole dipoles] for glucagon binding) or are hydrophobic (utilizing the hydrophobic effect). The [https://en.wikipedia.org/wiki/Active_site binding site] location of the hormone peptide ligand has been identified, and the N-terminus of glucagon is known to bind partly with the ECD while the rest of glucagon binds deep into the binding pocket. The [https://en.wikipedia.org/wiki/Amino_acid amino acids] at the N-terminus of the class B 7TM have the ability to form [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds] and [https://en.wikipedia.org/wiki/Ionic_bonding ionic interactions], which can be seen in the [https://en.wikipedia.org/wiki/Peptide_sequence amino acid sequence] of glucagon (Figure 5). <ref name="Sequence">PMID: 11946536</ref> | ||
| Line 39: | Line 39: | ||
[[Image:Glucagonstructure.png|(|):|360 px|right|thumb|'''Figure 5: Structure of Glucagon:''' The side chains of the residues making up glucagon are depicted. Coloration on the side chains indicate certain [https://en.wikipedia.org/wiki/Atom atoms] that determine the properties the residues hold. The blue indicates a [https://en.wikipedia.org/wiki/Nitrogen nitrogen] atom (hydrophilic properties), the green on the side chains indicates carbon atoms (non-polar hydrophobic properties), and the red coloration indicates an [https://en.wikipedia.org/wiki/Oxygen oxygen] atom (hydrophilic properties). [http://www.rcsb.org/pdb/home/home.do PDB] [http://www.rcsb.org/pdb/explore.do?structureId=1GCN 1GCN] ]] | [[Image:Glucagonstructure.png|(|):|360 px|right|thumb|'''Figure 5: Structure of Glucagon:''' The side chains of the residues making up glucagon are depicted. Coloration on the side chains indicate certain [https://en.wikipedia.org/wiki/Atom atoms] that determine the properties the residues hold. The blue indicates a [https://en.wikipedia.org/wiki/Nitrogen nitrogen] atom (hydrophilic properties), the green on the side chains indicates carbon atoms (non-polar hydrophobic properties), and the red coloration indicates an [https://en.wikipedia.org/wiki/Oxygen oxygen] atom (hydrophilic properties). [http://www.rcsb.org/pdb/home/home.do PDB] [http://www.rcsb.org/pdb/explore.do?structureId=1GCN 1GCN] ]] | ||
| - | Specific amino acid interactions have been identified in GCGR that maximize affinity. This includes the alpha helical structure of the <scene name='72/721535/Stalk/1'>stalk</scene>. The alpha helical structure of the stalk interacts directly with glucagon, as it extends nearly three helical turns above the membrane. When the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases. A [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] study mutating <scene name='72/721535/Ala135/1'>alanine 135</scene> to a proline. Proline disrupts helices. The Ala135Pro mutant had significant lower affinity for glucagon.<ref name=" | + | Specific amino acid interactions have been identified in GCGR that maximize affinity. This includes the alpha helical structure of the <scene name='72/721535/Stalk/1'>stalk</scene>. The alpha helical structure of the stalk interacts directly with glucagon, as it extends nearly three helical turns above the membrane. When the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases. A [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] study mutating <scene name='72/721535/Ala135/1'>alanine 135</scene> to a proline. Proline disrupts helices. The Ala135Pro mutant had significant lower affinity for glucagon.<ref name= "Siu 2013"/> Furthermore, there are certain interactions that hold the helices of the 7TM in the conformation that maximizes [http://www.chemicool.com/definition/affinity.html affinity]. <ref name="Ligands">PMID: 21542831</ref> The high affinity conformation of GCGR is the open conformation, when glucagon can bind. Without these specific interactions between the residues, the open conformation is not stabilized and GCGR remains in the closed conformation, where glucagon cannot bind.<ref name= "Siu 2013"/> The [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin_actual/2'>Cys 294 and Cys 224</scene> serves to hold the helices in the proper orientation for binding and stabilizes the open conformation. Additionally, the [https://en.wikipedia.org/wiki/Salt_bridge_%28protein_and_supramolecular%29 salt bridges] between |
<scene name='72/721535/Salt_bridge_residues/1'>Glu 406, Arg 173, and Arg 346</scene> hold the open conformation together for higher affinity. <ref name="Ligands">PMID: 21542831</ref> | <scene name='72/721535/Salt_bridge_residues/1'>Glu 406, Arg 173, and Arg 346</scene> hold the open conformation together for higher affinity. <ref name="Ligands">PMID: 21542831</ref> | ||
Revision as of 19:00, 9 June 2016
Glucagon G protein-coupled receptor
References
- ↑ 1.0 1.1 1.2 Zhang Y, Devries ME, Skolnick J. Structure modeling of all identified G protein-coupled receptors in the human genome. PLoS Comput Biol. 2006 Feb;2(2):e13. Epub 2006 Feb 17. PMID:16485037 doi:http://dx.doi.org/10.1371/journal.pcbi.0020013
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, Marshall FH. Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol. 2014 Jul;171(13):3132-45. doi: 10.1111/bph.12689. PMID:24628305 doi:http://dx.doi.org/10.1111/bph.12689
- ↑ 4.0 4.1 4.2 Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ 5.0 5.1 Hollenstein K, Kean J, Bortolato A, Cheng RK, Dore AS, Jazayeri A, Cooke RM, Weir M, Marshall FH. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013 Jul 25;499(7459):438-43. doi: 10.1038/nature12357. Epub 2013 Jul 17. PMID:23863939 doi:http://dx.doi.org/10.1038/nature12357
- ↑ 6.0 6.1 6.2 Yang L, Yang D, de Graaf C, Moeller A, West GM, Dharmarajan V, Wang C, Siu FY, Song G, Reedtz-Runge S, Pascal BD, Wu B, Potter CS, Zhou H, Griffin PR, Carragher B, Yang H, Wang MW, Stevens RC, Jiang H. Conformational states of the full-length glucagon receptor. Nat Commun. 2015 Jul 31;6:7859. doi: 10.1038/ncomms8859. PMID:26227798 doi:http://dx.doi.org/10.1038/ncomms8859
- ↑ 7.0 7.1 7.2 7.3 Miller LJ, Dong M, Harikumar KG. Ligand binding and activation of the secretin receptor, a prototypic family B G protein-coupled receptor. Br J Pharmacol. 2012 May;166(1):18-26. doi: 10.1111/j.1476-5381.2011.01463.x. PMID:21542831 doi:http://dx.doi.org/10.1111/j.1476-5381.2011.01463.x
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 'Lehninger A., Nelson D.N, & Cox M.M. (2008) Lehninger Principles of Biochemistry. W. H. Freeman, fifth edition.'
- ↑ 9.0 9.1 Yang DH, Zhou CH, Liu Q, Wang MW. Landmark studies on the glucagon subfamily of GPCRs: from small molecule modulators to a crystal structure. Acta Pharmacol Sin. 2015 Sep;36(9):1033-42. doi: 10.1038/aps.2015.78. Epub 2015, Aug 17. PMID:26279155 doi:http://dx.doi.org/10.1038/aps.2015.78
- ↑ Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009 May;8(5):369-85. doi: 10.1038/nrd2782. Epub 2009 Apr, 14. PMID:19365392 doi:http://dx.doi.org/10.1038/nrd2782
- ↑ Xu Y, Xie X. Glucagon receptor mediates calcium signaling by coupling to G alpha q/11 and G alpha i/o in HEK293 cells. J Recept Signal Transduct Res. 2009 Dec;29(6):318-25. doi:, 10.3109/10799890903295150. PMID:19903011 doi:http://dx.doi.org/10.3109/10799890903295150
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedstructure_article - ↑ Thomsen J, Kristiansen K, Brunfeldt K, Sundby F. The amino acid sequence of human glucagon. FEBS Lett. 1972 Apr 1;21(3):315-319. PMID:11946536
- ↑ 14.0 14.1 14.2 Weston C, Lu J, Li N, Barkan K, Richards GO, Roberts DJ, Skerry TM, Poyner D, Pardamwar M, Reynolds CA, Dowell SJ, Willars GB, Ladds G. Modulation of Glucagon Receptor Pharmacology by Receptor Activity-modifying Protein-2 (RAMP2). J Biol Chem. 2015 Sep 18;290(38):23009-22. doi: 10.1074/jbc.M114.624601. Epub, 2015 Jul 21. PMID:26198634 doi:http://dx.doi.org/10.1074/jbc.M114.624601
- ↑ 15.0 15.1 Wootten D, Lindmark H, Kadmiel M, Willcockson H, Caron KM, Barwell J, Drmota T, Poyner DR. Receptor activity modifying proteins (RAMPs) interact with the VPAC2 receptor and CRF1 receptors and modulate their function. Br J Pharmacol. 2013 Feb;168(4):822-34. doi: 10.1111/j.1476-5381.2012.02202.x. PMID:22946657 doi:http://dx.doi.org/10.1111/j.1476-5381.2012.02202.x
- ↑ 16.0 16.1 Lau J, Behrens C, Sidelmann UG, Knudsen LB, Lundt B, Sams C, Ynddal L, Brand CL, Pridal L, Ling A, Kiel D, Plewe M, Shi S, Madsen P. New beta-alanine derivatives are orally available glucagon receptor antagonists. J Med Chem. 2007 Jan 11;50(1):113-28. PMID:17201415 doi:http://dx.doi.org/10.1021/jm058026u