User:R. Jeremy Johnson/Glucagon Receptor
From Proteopedia
| Line 24: | Line 24: | ||
==== Intracellular Helix VIII ==== | ==== Intracellular Helix VIII ==== | ||
| - | The GCGR also contains an intracellular Helix VIII that is comprised of roughly 20 amino acids at the C-terminal end. This helix tilts approximately 25 degrees away from the membrane - the corresponding position in class A receptors are turned toward the membrane<ref name=" | + | The GCGR also contains an intracellular Helix VIII that is comprised of roughly 20 amino acids at the C-terminal end. This helix tilts approximately 25 degrees away from the membrane - the corresponding position in class A receptors are turned toward the membrane.<ref name= "Siu 2013"/> Although researchers are not entirely sure of its function, this helix is completely conserved in class B structures. |
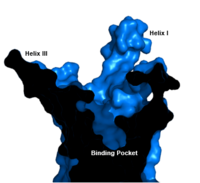
==== Binding Pocket ==== | ==== Binding Pocket ==== | ||
| Line 58: | Line 58: | ||
[https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G protein-coupled receptors] | [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G protein-coupled receptors] | ||
| - | |||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | </StructureSection> | ||
Revision as of 19:22, 9 June 2016
Glucagon G protein-coupled receptor
Class B GPCRsG protein coupled receptors (GPCRs) are the largest class of integral membrane proteins. GPCRs are divided into five families; the rhodopsin family (class A), the secretin family (class B), the glutamate family (class C), the frizzled/taste family (class F), and the adhesion family.[1] Roughly 5% of the human genome encodes g protein-coupled receptors, which are responsible for the transduction of endogenous signals and the instigation of cellular responses.[1] All GPCRs contain a similar seven α-helical transmembrane domain that once bound to its ligand, undergoes a conformational change and tranduces a signal to coupled, heterotrimeric G proteins. The initiation of intracellular signal pathways occur in response to stimuli such as light, Ca2+, amino acids, nucleotides, odorants, peptides, and other proteins, and accomplishes many interesting physiological roles. [1] The human glucagon receptor (GCGR) is one of 15 secretin-like, or Class B, G-protein coupled receptors (GPCRs). Like other GPCRs, it has a helical domain and a globular N-terminus (ECD).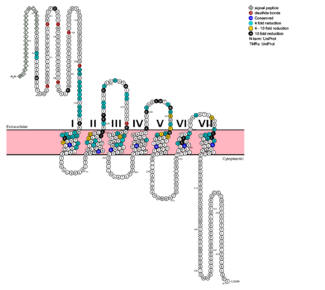 Figure 1: Snake Plot of GCGR TMD. Residues of particular importance in glucagon binding affinity are found in green, yellow, and black. Residues in red are the location of critical disulfide bonds, while blue residues were found to be highly conserved across all class B GPCRs.[2] Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases the intracellular concentration of cAMP, inositol phosphate, and calcium levels. [3] These secondary messengers are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest as disease targets. [4] Structural approaches to the development of agonists and antagonists have however been hampered by the lack of accurate Class B TMD visualizations. Recent crystal structure images of corticoptropin-releasing factor receptor 1 (PDB: 4K5Y) and human glucagon receptor (PDB: 4L6R) were accomplished through x-ray crystallography. [5][2] Structures of Class A vs. Class B GPCRsClass A vs. class B glucagon receptors share less than fifteen percent sequence homology, but both share a 7TM domain.[5] Understanding for class A family of GCGRs of the structure-function mechanism has made great progress over the past few years, but understanding of class B has fallen behind but is now catching up.[2] Comparison of the helices to that of the helices showed that the general orientation and positioning of the alpha helices are conserved through both classes. Detailed structural alignments of the two GPCR subclasses revealed multiple sequence misalignments in the transmembrane region signifying a variety of structural deviations in the transmembrane helices.[2] The N-terminal end of in class B GCGR, located in the 7TM, is longer than any known class A GPCR structure and stretches three supplementary helical turns above the extracellular (EC) membrane boundary. This region is referred to as the . The stalk is involved in glucagon binding and helps in defining the orientation of the ECD with respect to the 7TM domain.[2] Also specific to class B GPCRs, a Gly residue at position 393 induces a ; this bend is stabilized by the hydrophobic interaction between the . One of the most distinguishable characteristics of the class B 7TM is the of 25 degrees and its length compared to that of , which is much shorter. This helical tilt results from Glu 406 in helix VIII that is fully conserved in secretin-like receptors and forms two interhelical with conserved residues Arg 173 and Arg 346.[2] Despite these differences, a vital region that is conserved in both class B and class A receptors is the disulfide bond between in extracellular loop two (ECL2). This bond stabilizes the receptors entire 7TM fold. Lastly, the locations of the extracellular tips for class B glucagon receptors allow for a much wider and deeper ligand-binding pocket than any of the class A GPCRs.[2] While interface interactions between helices VI, V, and III, are not unique to class B receptors because certain homologous and even conserved residues exist in class A receptors (like Tyr 239 and Leu 358), as a part of the interface between helices VI, V, and III, a Class B-specific hydrogen bond occurs between Asn 318 of Helix V and Leu 242 of Helix III.[2] How These Structures Lead to Function Figure 2: Open conformation in contrast to the closed conformation. The movement of the single helix over the top of the transmembrane domain is the most distinguishable characteristic between closed and open conformation. The is not accessible to glucagon in the closed conformation.[6] Structurally, the 7TM and its signature seven helical structure is involved in signaling via coupling to heterotrimeric G proteins that activate adenylate cyclase to increase the levels of intracellular cyclic AMP. Additionally, this coupling increases levels of IP3 and intracellular calcium levels.[2] The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide receptors. [7] The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed conformation of the GCGR (Figure 2). The open conformation is when glucagon can bind to GCGR; in the closed conformation binding does not occur.[7] Glucagon Receptor (GCGR)The glucagon class B GPCR (GCGR) is involved in glucose homeostasis through the binding of the signal peptide glucagon. Glucagon is released from pancreatic α-cells when blood glucose levels fall after a period of fasting or several hours following intake of dietary carbohydrates.[8] Once the peptide hormone is released, it binds to GCGR, a 485 amino acid protein found in the liver, kidney, intestinal smooth muscle, brain, and adipose tissues. [9] Upon binding, signaling is initiated to heterotrimeric G-proteins containing Gαs. [10] GCGR can regulate additional signal pathways, including G-proteins of the Gαi family through the adoption of differing receptor conformations. [11] Glucagon's main role is the regulation of blood glucose levels.[8] Glucagon lowers the concentration of fructose 2,6-bisphosphate which is an allosteric inhibitor of the gluconeogenic enzyme fructose 1,6-bisphosphotase and activates phosphofructose kinase 1, which increases glucose levels via glycolysis.[8] Glucagon is also a regulator of the production of cholesterol, which is an energetically intensive process. When energy resources are low, downregulation of cholesterol production begins with glucagon binding to GCGR, which stimulates the phosphorylation of HMG-CoA.[8] HMG-CoA is inactivated by phosphorylation and moderates cholesterol production to conserve energy.[8] Glucagon also takes part in fatty acid mobilization by affecting levels of adipose tissue in the organism. Activation of GCGR by glucagon initiates triacylglycerol breakdown and the phosphorylation of perilipin and lipases via cAMP signal pathways.[8] This allows the body to export fatty acids to the liver and other crucial tissues for energy use and makes more glucose available for use in brain functioning.[8] GCGR-Specific TraitsHelix I Stalk RegionThe tip of Helix I extends above the cell membrane into the extracellular space creating a . This region is longer than any other class of GPCR and extends three α-helical turns above the plane of the membrane. It is proposed that the stalk helps to capture the glucagon peptide and facilitates it's insertion into the 7tm.[2] Intracellular Helix VIIIThe GCGR also contains an intracellular Helix VIII that is comprised of roughly 20 amino acids at the C-terminal end. This helix tilts approximately 25 degrees away from the membrane - the corresponding position in class A receptors are turned toward the membrane.[2] Although researchers are not entirely sure of its function, this helix is completely conserved in class B structures. Binding PocketThe class B GPCR has the widest and longest of all other classes of GPCRs. The distance between the EC tips of Helicies II and VI as well as between the tips of Helicies III and VII are some of the largest among the GPCRs.[2] As a result, the binding cavity of GCGR is located deeper inside the receptor, meaning glucagon binds much closer to the cell membrane.Other Unique Structural FeaturesAn important interface stabilization interaction between Helices I and VII occurs between Ser 152 of Helix I and Ser 390 of Helix VII. Due to their close proximity to one another, they form an important which stabilizes the structure of GCGR. Mutations to the homologous residues Ser 135 and Ser 392 have been shown to alter receptor signaling in glucagon-like peptide-1 receptor (GLP1R). Glucagon BindingThe large, soluble N-terminal extracellular domains (ECD) of GCGR provide initial ligand selectivity with the deep, ligand pocket of the TMD providing secondary recognition.[9] The active, or open conformation, is characterized by an intracellular outward movement of (breaking hydrogen bonds between and )[4] and an extracellular rotation of the ECD until it is almost perpendicular to the membrane surface.[6] While the stalk region of Helix I helps to facilitate the motion of the ECD, intracellular G-protein coupling and extracellular glucagon binding stabilized this active state. In the abscence of glucagon, however, the GCGR adopts a closed conformation in which all three of the extracellular loops of the 7tm () can interact with the ECD.[6] In this closed state, the ECD covers the extracellular surface of the 7tm. To transition between states, the ECD rotates and moves down towards the 7tm domain. This transition mechanism is consistent with the "two-domain" binding mechanism of class B GCPRs in which (1) the C-terminus of the ligand first binds to the ECD allowing (2) the N-terminus of the ligand to interact with the 7tm and activate the protein.[4]
The residues in the binding pocket that are in direct contact with the glucagon molecule are polar (utilizing the attraction of opposite charges or dipoles for glucagon binding) or are hydrophobic (utilizing the hydrophobic effect). The binding site location of the hormone peptide ligand has been identified, and the N-terminus of glucagon is known to bind partly with the ECD while the rest of glucagon binds deep into the binding pocket. The amino acids at the N-terminus of the class B 7TM have the ability to form hydrogen bonds and ionic interactions, which can be seen in the amino acid sequence of glucagon (Figure 5). [12] 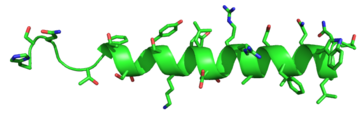 Figure 5: Structure of Glucagon: The side chains of the residues making up glucagon are depicted. Coloration on the side chains indicate certain atoms that determine the properties the residues hold. The blue indicates a nitrogen atom (hydrophilic properties), the green on the side chains indicates carbon atoms (non-polar hydrophobic properties), and the red coloration indicates an oxygen atom (hydrophilic properties). PDB 1GCN Specific amino acid interactions have been identified in GCGR that maximize affinity. This includes the alpha helical structure of the . The alpha helical structure of the stalk interacts directly with glucagon, as it extends nearly three helical turns above the membrane. When the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases. A mutagenesis study mutating to a proline. Proline disrupts helices. The Ala135Pro mutant had significant lower affinity for glucagon.[2] Furthermore, there are certain interactions that hold the helices of the 7TM in the conformation that maximizes affinity. [7] The high affinity conformation of GCGR is the open conformation, when glucagon can bind. Without these specific interactions between the residues, the open conformation is not stabilized and GCGR remains in the closed conformation, where glucagon cannot bind.[2] The disulfide bond between serves to hold the helices in the proper orientation for binding and stabilizes the open conformation. Additionally, the salt bridges between hold the open conformation together for higher affinity. [7] Mutagenesis and photo cross-linking studies determined essential, conserved residues in glucagon and have been in red.[2] Glucagon residues His 1, Gln 3, Phe 6, and Tyr 10 are critical to successful binding interaction with the GCGR while others are important for structural rigidity. The n-terminus of glucagon (Fig. 3) leads to a protuberance that fits into the deep, interior cavity of the GCGR 7TMD (Fig. 2) where four residues reside that play strong roles in ligand binding affinity. There is a to the entrance of the cavity, providing a firm anchor during peptide docking. (also see Fig. 2) Glucagon Signaling PathwayGlucagon binds to the open conformation of GCGR; the GCGR located on the plasma membrane. Glucagon binding to GCGR induces a conformational change in GCGR. This conformation change induces the active state of the protein. The active state of the protein exchanges a guanosine diphosphate (GDP) for guanosine triphosphate (GTP) that is bound to the alpha subunit. With the GTP in place, the activated alpha subunit dissociates from the heterotrimeric G protein'sbeta and gamma subunits. Following dissociation, the alpha subunit can activate adenylate cyclase. Activated adenylate cyclase, catalyzes the conversion of adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP). cAMP then serves as a secondary messenger to activate, through allosteric binding, cAMP dependent protein kinase A (PKA). PKA activates via phosphorylation the phosphorylase b kinase. The phosphorylase b kinase phosphorylates glycogen phosphorylase b to convert to the active form, phosphorylase a. Phosphorylase a finally catalyzes the release of glucose-1-phosphate into the bloodstream from glycogen polymers (Figure 6). 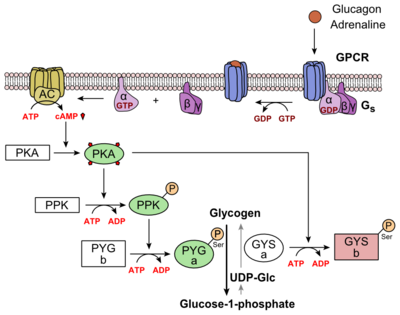 Figure 6: Metabolic Regulation of Glycogen by Glucagon.Depicted is the visualization of the glucagon signaling pathway through the GCGR. The location of the GCGR, the release of the alpha subunit from the beta and gamma subunits, and the enzyme cascade to result in the releasing of glucose are depicted. Abbreviations for the enzymes in the cascade include- PPK: phosphorylase kinase; PYG b: glycogen phosphorylase b; PYG a: glycogen phosphorylase a. Clinical relevanceBecause GCGR can interact with multiple types of G protein subfamilies, discovering small molecule inhibitors could lead to a wide range of focused therapies.[13] Blocking conformations that favor interaction with specific G proteins could allow the knockdown of targeted signal pathways. For example, GCGR is known to interact with inhibitory Gαi proteins that antagonize cAMP production.[13] The finding of an agonist for this pathway could lead to breakthroughs in the treatment of diabetes mellitus. Recently some fundamental work has been done with RAMPs which were shown to alter ligand preference in class B GPCRs.[14] Specifically, RAMP2 association has been shown to alter the pharmacology of all GCGR ligands (glucagon and oxyntomodulin). RAMP2 association altered cAMP production, indicating an effect on signaling bias and g protein coupling.[14] Attempts to target the GCGR have proven relatively unsuccessful. Three small molecule modulators were reported with the hope of enhanced pharmaceutical regulation.[15] (Fig. 4) It is not clear if this work has resulted in additional pharmacological modalities, but any progress has been modest, at best. Some gains have been made in targeting glucagon-like peptide-1 receptors (a GPCR closely related to GCGR) but with the caveat of severe, adverse side-effects.[13] Encouraging results have recently come from Eli Lilly and Company who have been testing a small molecule antagonist of the GCGR (LY2409021) in phase two trials with some success, providing hope for some more specific control of diabetes mellitus.[15] In addition to diabetes mellitus, future development of signal bias modulators promise to provide focused therapies for obesity and heart disease, as well as related secondary pathological conditions such as hypertension and cancer. See AlsoPSI Structural Biology Database References
| ||||||||||||