User:R. Jeremy Johnson/Glucagon Receptor
From Proteopedia
< User:R. Jeremy Johnson(Difference between revisions)
| Line 3: | Line 3: | ||
==Class B GPCRs== | ==Class B GPCRs== | ||
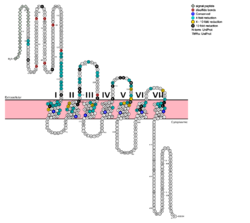
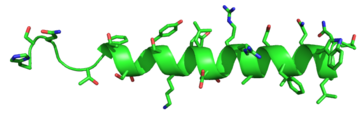
| - | G protein coupled receptors (GPCRs) are the largest class of integral membrane proteins. GPCRs are divided into five families; the rhodopsin family (class A), the secretin family (class B), the glutamate family (class C), the frizzled/taste family (class F), and the adhesion family.<ref name= "Zhang 2006"/> Roughly 5% of the human genome encodes g protein-coupled receptors, which are responsible for the transduction of endogenous signals and the instigation of cellular responses.<ref name= "Zhang 2006"/> All GPCRs contain a similar seven α-helical transmembrane domain <scene name='72/727091/Full_Structure_with_Labels/1'>(TMD or 7TMD)</scene> that once bound to its ligand, undergoes a conformational change and tranduces a signal to coupled, heterotrimeric G proteins. The initiation of intracellular signal pathways occur in response to stimuli such as light, Ca<sup>2+</sup>, amino acids, nucleotides, odorants, peptides, and other proteins, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor#Physiological_roles and accomplishes many interesting physiological roles]. <ref name= "Zhang 2006">DOI 10.1371/journal.pcbi.0020013</ref> The '''human glucagon receptor''' ('''GCGR''') is one of 15 secretin-like, or Class B, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptors] (GPCRs). Like other GPCRs, it has a <scene name='72/721538/7tm_labeled_helicies/3'>7 trans-membrane </scene> helical domain and a globular N-terminus <scene name='72/721538/Ecd/2'>extracellular domain</scene> (ECD). [[Image:Protter GLR HUMAN.png | | + | G protein coupled receptors (GPCRs) are the largest class of integral membrane proteins. GPCRs are divided into five families; the rhodopsin family (class A), the secretin family (class B), the glutamate family (class C), the frizzled/taste family (class F), and the adhesion family.<ref name= "Zhang 2006"/> Roughly 5% of the human genome encodes g protein-coupled receptors, which are responsible for the transduction of endogenous signals and the instigation of cellular responses.<ref name= "Zhang 2006"/> All GPCRs contain a similar seven α-helical transmembrane domain <scene name='72/727091/Full_Structure_with_Labels/1'>(TMD or 7TMD)</scene> that once bound to its ligand, undergoes a conformational change and tranduces a signal to coupled, heterotrimeric G proteins. The initiation of intracellular signal pathways occur in response to stimuli such as light, Ca<sup>2+</sup>, amino acids, nucleotides, odorants, peptides, and other proteins, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor#Physiological_roles and accomplishes many interesting physiological roles]. <ref name= "Zhang 2006">DOI 10.1371/journal.pcbi.0020013</ref> The '''human glucagon receptor''' ('''GCGR''') is one of 15 secretin-like, or Class B, [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor G-protein coupled receptors] (GPCRs). Like other GPCRs, it has a <scene name='72/721538/7tm_labeled_helicies/3'>7 trans-membrane </scene> helical domain and a globular N-terminus <scene name='72/721538/Ecd/2'>extracellular domain</scene> (ECD). [[Image:Protter GLR HUMAN.png |250 px|left|thumb|'''Figure 1''': Snake Plot of GCGR TMD. Residues of particular importance in glucagon binding affinity are found in green, yellow, and black. Residues in red are the location of critical disulfide bonds, while blue residues were found to be highly conserved across all class B GPCRs.<ref name= "Siu 2013"/>]] |
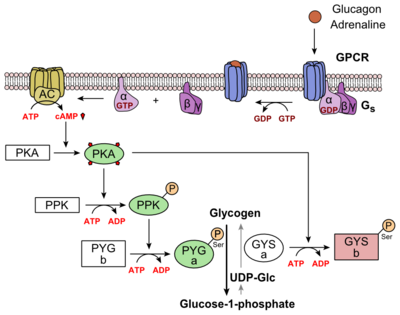
Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases the intracellular concentration of cAMP, inositol phosphate, and calcium levels. <ref>DOI 10.1111/bph.12689</ref> These secondary messengers are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest as disease targets. <ref name= "Hollenstein 2014">DOI 10.1016/j.tips.2013.11.001</ref> Structural approaches to the development of agonists and antagonists have however been hampered by the lack of accurate Class B TMD visualizations. Recent crystal structures of corticoptropin-releasing factor receptor 1 (PDB: 4K5Y) and human glucagon receptor (PDB: 4L6R) provide a comparison to more well-studied class A GPCRs. <ref name= "Hollenstein 2013">DOI 10.1038/nature12357</ref><ref name= "Siu 2013">DOI 10.1038/nature12393</ref> | Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases the intracellular concentration of cAMP, inositol phosphate, and calcium levels. <ref>DOI 10.1111/bph.12689</ref> These secondary messengers are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest as disease targets. <ref name= "Hollenstein 2014">DOI 10.1016/j.tips.2013.11.001</ref> Structural approaches to the development of agonists and antagonists have however been hampered by the lack of accurate Class B TMD visualizations. Recent crystal structures of corticoptropin-releasing factor receptor 1 (PDB: 4K5Y) and human glucagon receptor (PDB: 4L6R) provide a comparison to more well-studied class A GPCRs. <ref name= "Hollenstein 2013">DOI 10.1038/nature12357</ref><ref name= "Siu 2013">DOI 10.1038/nature12393</ref> | ||
| Line 11: | Line 11: | ||
===How These Structures Lead to Function=== | ===How These Structures Lead to Function=== | ||
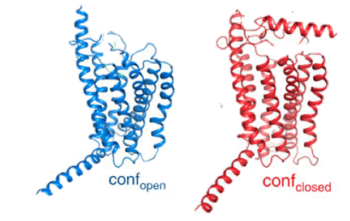
| - | [[Image:Closed and open conformation.png|(|):| | + | [[Image:Closed and open conformation.png|(|):|350 px|center|thumb|'''Figure 2: Open conformation in contrast to the closed conformation.''' The movement of the single helix over the top of the transmembrane domain is the most distinguishable characteristic between closed and open conformation. The <scene name='72/721535/Stalk/1'>stalk</scene> is not accessible to glucagon in the closed conformation.<ref name="Lastt">PMID: 26227798</ref>]] |
Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels.<ref name= "Siu 2013"/> The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide [https://en.wikipedia.org/wiki/Receptor_(biochemistry) receptors]. <ref name="Ligands">PMID: 21542831</ref> The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed [https://en.wikipedia.org/wiki/Conformation conformation] of the GCGR (Figure 2). The open conformation is when glucagon can bind to GCGR; in the closed conformation binding does not occur.<ref name="Ligands">PMID: 21542831</ref> | Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels.<ref name= "Siu 2013"/> The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide [https://en.wikipedia.org/wiki/Receptor_(biochemistry) receptors]. <ref name="Ligands">PMID: 21542831</ref> The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed [https://en.wikipedia.org/wiki/Conformation conformation] of the GCGR (Figure 2). The open conformation is when glucagon can bind to GCGR; in the closed conformation binding does not occur.<ref name="Ligands">PMID: 21542831</ref> | ||
Current revision
Glucagon G protein-coupled receptor
Student Contributors
Steven Bennett
Sydney Caskey
Alexis Coulis
Olivia Murfield
Allie Paton
Dean Williams