We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Myosin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
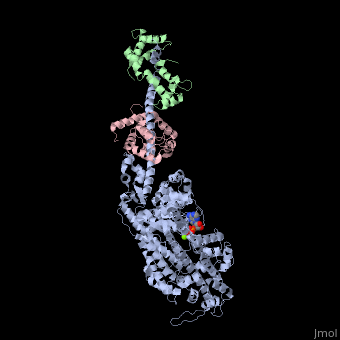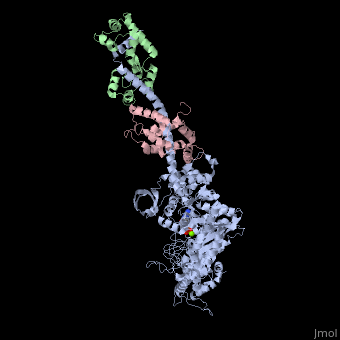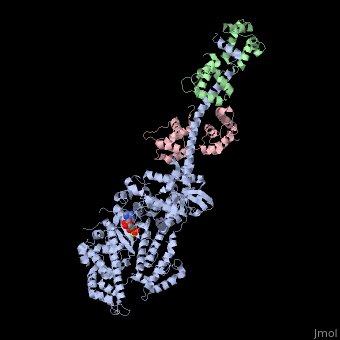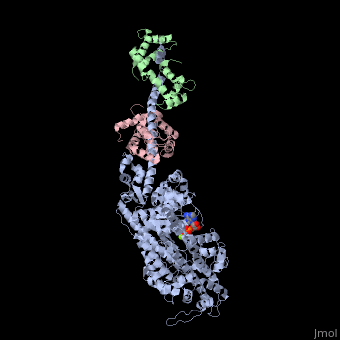
| - | + | <StructureSection load='3i5f' size='350' side='right' caption='Squid myosin II: heavy chain (grey), regulatory light chain (green), catalytic light chain (pink) complex with ADP (stick model) and Mg+2 ion (green) [[3i5f]]' scene=''> | |
== Introduction == | == Introduction == | ||
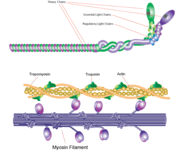
[[Myosin]] is one of three major classes of molecular motor proteins: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. <ref name="Spudich">PMID: 8824453 </ref> Myosin is a superfamily of proteins which bind actin, hydrolyze ATP and transduce force. Thus most are located in muscle cells. Composed of head, neck and tail domains. Head domain binds the actin and moves along it. The neck is a linker and binds the light chains which have a regulatory function. The tail interacts with cargo molecules (CBD)m. There are 18 classes of myosin.<br /> | [[Myosin]] is one of three major classes of molecular motor proteins: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. <ref name="Spudich">PMID: 8824453 </ref> Myosin is a superfamily of proteins which bind actin, hydrolyze ATP and transduce force. Thus most are located in muscle cells. Composed of head, neck and tail domains. Head domain binds the actin and moves along it. The neck is a linker and binds the light chains which have a regulatory function. The tail interacts with cargo molecules (CBD)m. There are 18 classes of myosin.<br /> | ||
| Line 38: | Line 38: | ||
==Disease== | ==Disease== | ||
Mutations in MIIA cause early onset myopathy<ref>PMID:20418530</ref>. Mutations in MVIIA cause Usher syndrome<ref>PMID:7870171</ref>. | Mutations in MIIA cause early onset myopathy<ref>PMID:20418530</ref>. Mutations in MVIIA cause Usher syndrome<ref>PMID:7870171</ref>. | ||
| + | </StructureSection> | ||
== 3D Structures of Myosin == | == 3D Structures of Myosin == | ||
Revision as of 06:02, 26 July 2016
| |||||||||||
3D Structures of Myosin
Updated on 26-July-2016
References
- ↑ Spudich JA, Finer J, Simmons B, Ruppel K, Patterson B, Uyeda T. Myosin structure and function. Cold Spring Harb Symp Quant Biol. 1995;60:783-91. PMID:8824453
- ↑ Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005 Jul;15(7):371-7. PMID:15935670 doi:http://dx.doi.org/10.1016/j.tcb.2005.05.004
- ↑ Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE. Myosin-V is a processive actin-based motor. Nature. 1999 Aug 5;400(6744):590-3. PMID:10448864 doi:http://dx.doi.org/10.1038/23072
- ↑ Buss F, Spudich G, Kendrick-Jones J. Myosin VI: cellular functions and motor properties. Annu Rev Cell Dev Biol. 2004;20:649-76. PMID:15473855 doi:http://dx.doi.org/10.1146/annurev.cellbio.20.012103.094243
- ↑ Hasson T, Skowron JF, Gilbert DJ, Avraham KB, Perry WL, Bement WM, Anderson BL, Sherr EH, Chen ZY, Greene LA, Ward DC, Corey DP, Mooseker MS, Copeland NG, Jenkins NA. Mapping of unconventional myosins in mouse and human. Genomics. 1996 Sep 15;36(3):431-9. PMID:8884266 doi:http://dx.doi.org/10.1006/geno.1996.0488
- ↑ Cox D, Berg JS, Cammer M, Chinegwundoh JO, Dale BM, Cheney RE, Greenberg S. Myosin X is a downstream effector of PI(3)K during phagocytosis. Nat Cell Biol. 2002 Jul;4(7):469-77. PMID:12055636 doi:http://dx.doi.org/10.1038/ncb805
- ↑ Tamura K, Iwabuchi K, Fukao Y, Kondo M, Okamoto K, Ueda H, Nishimura M, Hara-Nishimura I. Myosin XI-i links the nuclear membrane to the cytoskeleton to control nuclear movement and shape in Arabidopsis. Curr Biol. 2013 Sep 23;23(18):1776-81. doi: 10.1016/j.cub.2013.07.035. Epub 2013 , Aug 22. PMID:23973298 doi:http://dx.doi.org/10.1016/j.cub.2013.07.035
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:8316857
- ↑ 9.0 9.1 9.2 Nelson, D. and Cox, M.(2005). Lehninger Principles of Biochemistry. 4th ed. p.1119.
- ↑ Tajsharghi H, Hilton-Jones D, Raheem O, Saukkonen AM, Oldfors A, Udd B. Human disease caused by loss of fast IIa myosin heavy chain due to recessive MYH2 mutations. Brain. 2010 May;133(Pt 5):1451-9. doi: 10.1093/brain/awq083. PMID:20418530 doi:http://dx.doi.org/10.1093/brain/awq083
- ↑ Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al.. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995 Mar 2;374(6517):60-1. PMID:7870171 doi:http://dx.doi.org/10.1038/374060a0
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, David Canner, Laurel Koopmans, Jaime Prilusky