This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Phosphotransferase
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
== Structural highlights == | == Structural highlights == | ||
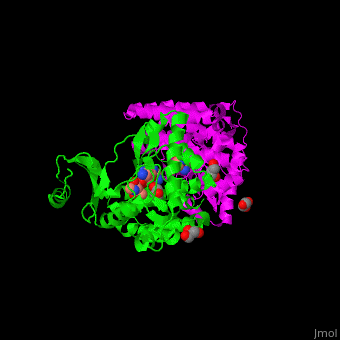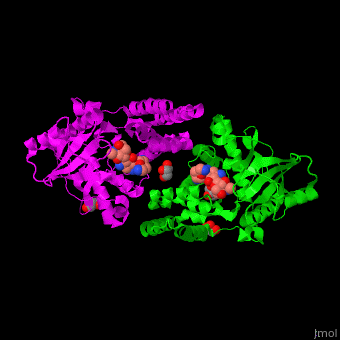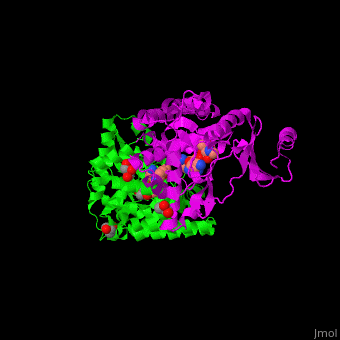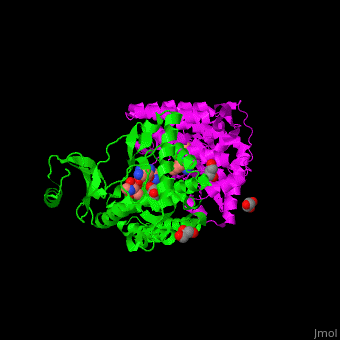
| - | The active site of amino glycoside PT is located at a structural cleft and contains the aspartate catalytic base<ref>PMID:19429619</ref>. | + | The <scene name='45/456502/Cv/2'>active site of amino glycoside PT</scene> is located at a structural cleft and contains the <scene name='45/456502/Cv/3'>aspartate catalytic base</scene><ref>PMID:19429619</ref>. Water molecules shown as red spheres. |
</StructureSection> | </StructureSection> | ||
Revision as of 12:42, 28 August 2016
| |||||||||||
3D structures of phosphotransferase
Updated on 28-August-2016
References
- ↑ Wright GD, Thompson PR. Aminoglycoside phosphotransferases: proteins, structure, and mechanism. Front Biosci. 1999 Jan 1;4:D9-21. PMID:9872733
- ↑ Mizrachi Nebenzahl Y, Blau K, Kushnir T, Shagan M, Portnoi M, Cohen A, Azriel S, Malka I, Adawi A, Kafka D, Dotan S, Guterman G, Troib S, Fishilevich T, Gershoni JM, Braiman A, Mitchell AM, Mitchell TJ, Porat N, Goliand I, Chalifa Caspi V, Swiatlo E, Tal M, Ellis R, Elia N, Dagan R. Streptococcus pneumoniae Cell-Wall-Localized Phosphoenolpyruvate Protein Phosphotransferase Can Function as an Adhesin: Identification of Its Host Target Molecules and Evaluation of Its Potential as a Vaccine. PLoS One. 2016 Mar 18;11(3):e0150320. doi: 10.1371/journal.pone.0150320., eCollection 2016. PMID:26990554 doi:http://dx.doi.org/10.1371/journal.pone.0150320
- ↑ Trach K, Burbulys D, Strauch M, Wu JJ, Dhillon N, Jonas R, Hanstein C, Kallio P, Perego M, Bird T, et al.. Control of the initiation of sporulation in Bacillus subtilis by a phosphorelay. Res Microbiol. 1991 Sep-Oct;142(7-8):815-23. PMID:1664534
- ↑ Young PG, Walanj R, Lakshmi V, Byrnes LJ, Metcalf P, Baker EN, Vakulenko SB, Smith CA. The crystal structures of substrate and nucleotide complexes of Enterococcus faecium aminoglycoside-2-phosphotransferase-IIa [APH(2)-IIa] provide insights into substrate selectivity in the APH(2) subfamily. J Bacteriol. 2009 Jul;191(13):4133-43. Epub 2009 May 8. PMID:19429619 doi:10.1128/JB.00149-09