We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Shikimate dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
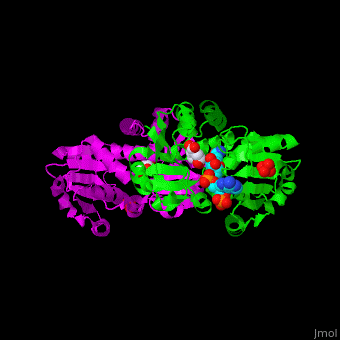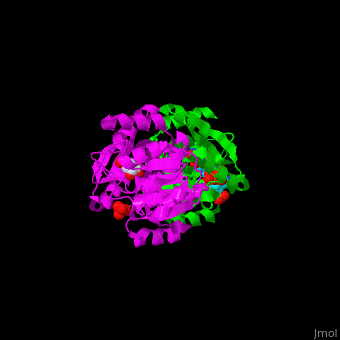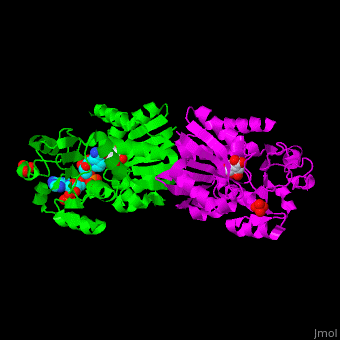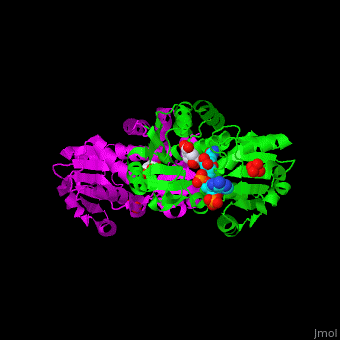
AroE changes its conformation from an open to closed one upon binding of the cofactor NADP. The active site contains the substrate shikimate<ref>PMID:17825835</ref>. | AroE changes its conformation from an open to closed one upon binding of the cofactor NADP. The active site contains the substrate shikimate<ref>PMID:17825835</ref>. | ||
*<scene name='54/540162/Cv/2'>NADP binding site</scene>. Water molecules shown as red spheres. | *<scene name='54/540162/Cv/2'>NADP binding site</scene>. Water molecules shown as red spheres. | ||
| - | *<scene name='54/540162/Cv/3'>Shikimate binding site</scene>. | ||
*<scene name='54/540162/Cv/4'>Whole binding site</scene>. | *<scene name='54/540162/Cv/4'>Whole binding site</scene>. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 12:31, 29 August 2016
| |||||||||||
3D Structures of shikimate dehydrogenase
Updated on 29-August-2016
References
- ↑ Singh S, Stavrinides J, Christendat D, Guttman DS. A phylogenomic analysis of the shikimate dehydrogenases reveals broadscale functional diversification and identifies one functionally distinct subclass. Mol Biol Evol. 2008 Oct;25(10):2221-32. doi: 10.1093/molbev/msn170. Epub 2008 Jul, 31. PMID:18669580 doi:http://dx.doi.org/10.1093/molbev/msn170
- ↑ Bagautdinov B, Kunishima N. Crystal structures of shikimate dehydrogenase AroE from Thermus thermophilus HB8 and its cofactor and substrate complexes: insights into the enzymatic mechanism. J Mol Biol. 2007 Oct 19;373(2):424-38. Epub 2007 Aug 21. PMID:17825835 doi:10.1016/j.jmb.2007.08.017
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Sohail Jooma