Phosphoserine aminotransferase
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structural highlights == | == Structural highlights == | ||
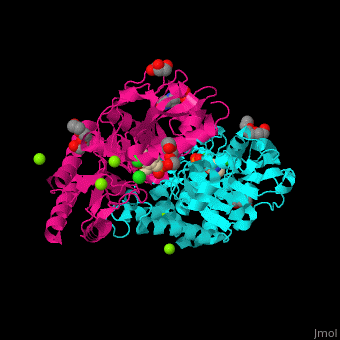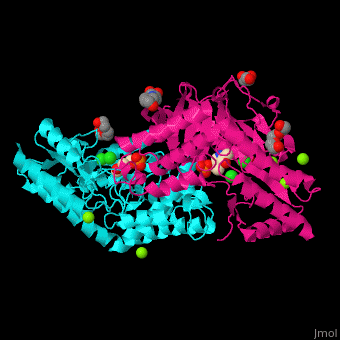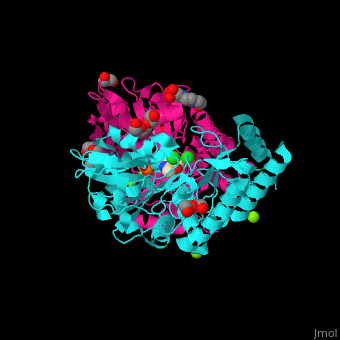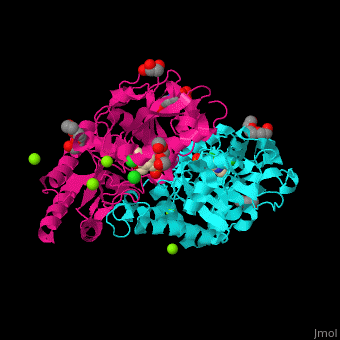
| - | PSAT overall structure shows a <scene name='48/488518/Cv/3'>small domain and a large one</scene>. The <scene name='48/488518/Cv/5'>active site</scene> contains the <scene name='48/488518/Cv/ | + | PSAT overall structure shows a <scene name='48/488518/Cv/3'>small domain and a large one</scene>. The <scene name='48/488518/Cv/5'>active site</scene> contains the <scene name='48/488518/Cv/7'>PLP co-factor bound to lysine side chain</scene><ref>PMID:15608117</ref>. Water molecules shown as red spheres. |
</StructureSection> | </StructureSection> | ||
Revision as of 10:40, 30 August 2016
| |||||||||||
3D structures of phosphoserine aminotransferase
Updated on 30-August-2016
References
- ↑ Basurko MJ, Marche M, Darriet M, Cassaigne A. Phosphoserine aminotransferase, the second step-catalyzing enzyme for serine biosynthesis. IUBMB Life. 1999 Nov;48(5):525-9. PMID:10637769 doi:http://dx.doi.org/10.1080/713803557
- ↑ Vie N, Copois V, Bascoul-Mollevi C, Denis V, Bec N, Robert B, Fraslon C, Conseiller E, Molina F, Larroque C, Martineau P, Del Rio M, Gongora C. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol Cancer. 2008 Jan 25;7:14. doi: 10.1186/1476-4598-7-14. PMID:18221502 doi:http://dx.doi.org/10.1186/1476-4598-7-14
- ↑ Dubnovitsky AP, Kapetaniou EG, Papageorgiou AC. Enzyme adaptation to alkaline pH: atomic resolution (1.08 A) structure of phosphoserine aminotransferase from Bacillus alcalophilus. Protein Sci. 2005 Jan;14(1):97-110. PMID:15608117 doi:14/1/97