We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Transposase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
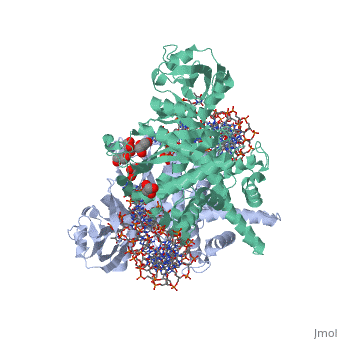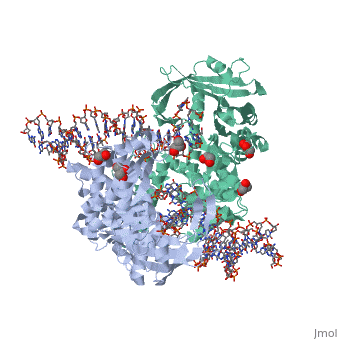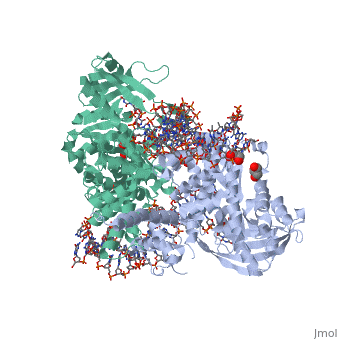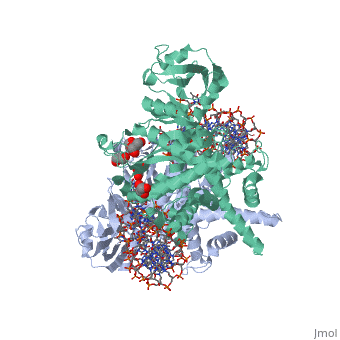
| - | <StructureSection load=' | + | <StructureSection load='3ecp' size='340' side='right' caption='Structure of Tn5 transposase complex with DNA (PDB code [[3ecp]]).' scene=''> |
== Function == | == Function == | ||
| - | '''Transposases''' bind to the ends of a transposon and catalyze its movement to another part of the genome by a cut-and-paste mechanism<ref>PMID:19478801</ref>. | + | '''Transposases''' bind to the ends of a transposon and catalyze its movement to another part of the genome by a cut-and-paste mechanism<ref>PMID:19478801</ref>. <br /> |
*'''Mu transposase''' (MuA) is essential for integration, replication-transposition and excision of bacteriophage Mu DNA into multiple sites of bacterial genome. Mu transposition occurs within the transposome which is a protein-DNA complex which includes 4 subunits of MuA<ref>PMID:7628012</ref>.<br /> | *'''Mu transposase''' (MuA) is essential for integration, replication-transposition and excision of bacteriophage Mu DNA into multiple sites of bacterial genome. Mu transposition occurs within the transposome which is a protein-DNA complex which includes 4 subunits of MuA<ref>PMID:7628012</ref>.<br /> | ||
*'''Hermes transposase''' is a fly protein<ref>PMID:19450689</ref>.<br /> | *'''Hermes transposase''' is a fly protein<ref>PMID:19450689</ref>.<br /> | ||
| Line 10: | Line 10: | ||
*'''Mos1 transposase''' mediates the movement of Mos1 transposon<ref>PMID:10762422</ref><br /> | *'''Mos1 transposase''' mediates the movement of Mos1 transposon<ref>PMID:10762422</ref><br /> | ||
*'''Tc3 transposase''' mediates the movement of Tc3 transposon in ''C. elegans''<ref>PMID:7838706</ref><br /> | *'''Tc3 transposase''' mediates the movement of Tc3 transposon in ''C. elegans''<ref>PMID:7838706</ref><br /> | ||
| - | |||
| - | == Disease == | ||
| - | |||
| - | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
| + | Transposase contains a DDE motif in its active site<ref>PMID:18790806</ref> enabling it to coordinate the binding of divalent metal ions. The metal ions affect transposase reactivity. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 10:02, 21 September 2016
| |||||||||||
3D structures of transposase
Updated on 21-September-2016
References
- ↑ Ivics Z, Li MA, Mates L, Boeke JD, Nagy A, Bradley A, Izsvak Z. Transposon-mediated genome manipulation in vertebrates. Nat Methods. 2009 Jun;6(6):415-22. doi: 10.1038/nmeth.1332. PMID:19478801 doi:http://dx.doi.org/10.1038/nmeth.1332
- ↑ Rice P, Mizuuchi K. Structure of the bacteriophage Mu transposase core: a common structural motif for DNA transposition and retroviral integration. Cell. 1995 Jul 28;82(2):209-20. PMID:7628012
- ↑ Park JM, Evertts AG, Levin HL. The Hermes transposon of Musca domestica and its use as a mutagen of Schizosaccharomyces pombe. Methods. 2009 Nov;49(3):243-7. doi: 10.1016/j.ymeth.2009.05.004. Epub 2009 May, 18. PMID:19450689 doi:http://dx.doi.org/10.1016/j.ymeth.2009.05.004
- ↑ Reznikoff WS. Transposon Tn5. Annu Rev Genet. 2008;42:269-86. PMID:18680433 doi:http://dx.doi.org/10.1146/annurev.genet.42.110807.091656
- ↑ Wang W, Swevers L, Iatrou K. Mariner (Mos1) transposase and genomic integration of foreign gene sequences in Bombyx mori cells. Insect Mol Biol. 2000 Apr;9(2):145-55. PMID:10762422
- ↑ Colloms SD, van Luenen HG, Plasterk RH. DNA binding activities of the Caenorhabditis elegans Tc3 transposase. Nucleic Acids Res. 1994 Dec 25;22(25):5548-54. PMID:7838706
- ↑ Klenchin VA, Czyz A, Goryshin IY, Gradman R, Lovell S, Rayment I, Reznikoff WS. Phosphate coordination and movement of DNA in the Tn5 synaptic complex: role of the (R)YREK motif. Nucleic Acids Res. 2008 Oct;36(18):5855-62. Epub 2008 Sep 12. PMID:18790806 doi:10.1093/nar/gkn577