5jk7
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | '''Unreleased structure''' | ||
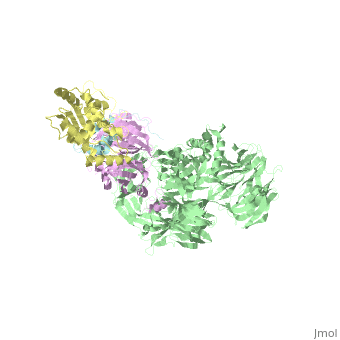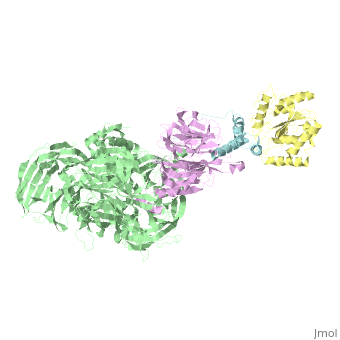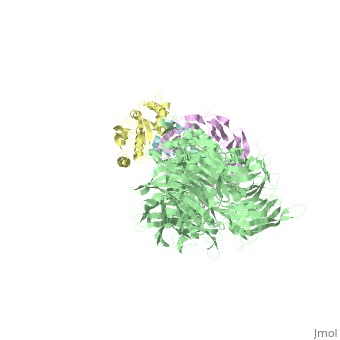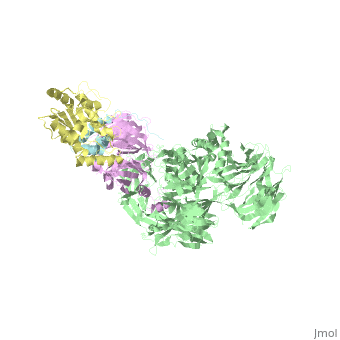
| - | The | + | ==The X-ray structure of the DDB1-DCAF1-Vpr-UNG2 complex== |
| + | <StructureSection load='5jk7' size='340' side='right' caption='[[5jk7]], [[Resolution|resolution]] 3.49Å' scene=''> | ||
| + | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>[[5jk7]] is a 8 chain structure. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=5JK7 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=5JK7 FirstGlance]. <br> | ||
| + | </td></tr><tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Non-specific_serine/threonine_protein_kinase Non-specific serine/threonine protein kinase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.7.11.1 2.7.11.1] </span></td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=5jk7 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=5jk7 OCA], [http://pdbe.org/5jk7 PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=5jk7 RCSB], [http://www.ebi.ac.uk/pdbsum/5jk7 PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=5jk7 ProSAT]</span></td></tr> | ||
| + | </table> | ||
| + | == Disease == | ||
| + | [[http://www.uniprot.org/uniprot/UNG_HUMAN UNG_HUMAN]] Defects in UNG are a cause of immunodeficiency with hyper-IgM type 5 (HIGM5) [MIM:[http://omim.org/entry/608106 608106]]. A rare immunodeficiency syndrome characterized by normal or elevated serum IgM levels with absence of IgG, IgA, and IgE. It results in a profound susceptibility to bacterial infections.<ref>PMID:12958596</ref> <ref>PMID:15967827</ref> | ||
| + | == Function == | ||
| + | [[http://www.uniprot.org/uniprot/VPR_HV1N5 VPR_HV1N5]] Involved in the transport of the viral pre-integration (PIC) complex to the nucleus during the early stages of the infection. This function is crucial for viral infection of non-dividing macrophages. May interact with karyopherin alpha/KPNA1 and KPNA2 to increase their affinity for proteins containing basic-type nuclear localization signal, including the viral matrix protein MA, thus facilitating the translocation of the viral genome into the nucleus. May also act directly at the nuclear pore complex, by binding nucleoporins phenylalanine-glycine (FG)-repeat regions (By similarity). May target specific host proteins for degradation by the 26S proteasome. Acts by associating with the cellular CUL4A-DDB1 E3 ligase complex through direct interaction with host VPRPB/DCAF-1. This change in the E3 ligase substrate specificity would result in cell cycle arrest or apoptosis in infected cells. Prevents infected cells from undergoing mitosis and proliferating, by inducing arrest or delay in the G2 phase of the cell cycle. This arrest creates a favorable environment for maximizing viral expression and production by rendering the HIV-1 LTR transcriptionally more active. In this context, Vpr stimulates gene expression driven by the HIV-1 LTR by interacting with human SP1, TFIIB and TFIID. Cell cycle arrest reportedly occurs within hours of infection and is not blocked by antiviral agents, suggesting that it is initiated by the Vpr carried into the virion. Additionally, Vpr induces apoptosis in a cell cycle dependent manner suggesting that these two effects are mechanistically linked. Interacts with mitochondrial permeability transition pore complex (PTPC). This interaction induces a rapid dissipation of the mitochondrial transmembrane potential, and mitochondrial release of apoptogenic proteins such as cytochrome C or apoptosis inducing factors. Detected in the serum and cerebrospinal fluid of AIDS patient, Vpr may also induce cell death to bystander cells (By similarity). [[http://www.uniprot.org/uniprot/DDB1_HUMAN DDB1_HUMAN]] Required for DNA repair. Binds to DDB2 to form the UV-damaged DNA-binding protein complex (the UV-DDB complex). The UV-DDB complex may recognize UV-induced DNA damage and recruit proteins of the nucleotide excision repair pathway (the NER pathway) to initiate DNA repair. The UV-DDB complex preferentially binds to cyclobutane pyrimidine dimers (CPD), 6-4 photoproducts (6-4 PP), apurinic sites and short mismatches. Also appears to function as a component of numerous distinct DCX (DDB1-CUL4-X-box) E3 ubiquitin-protein ligase complexes which mediate the ubiquitination and subsequent proteasomal degradation of target proteins. The functional specificity of the DCX E3 ubiquitin-protein ligase complex is determined by the variable substrate recognition component recruited by DDB1. DCX(DDB2) (also known as DDB1-CUL4-ROC1, CUL4-DDB-ROC1 and CUL4-DDB-RBX1) may ubiquitinate histone H2A, histone H3 and histone H4 at sites of UV-induced DNA damage. The ubiquitination of histones may facilitate their removal from the nucleosome and promote subsequent DNA repair. DCX(DDB2) also ubiquitinates XPC, which may enhance DNA-binding by XPC and promote NER. DCX(DTL) plays a role in PCNA-dependent polyubiquitination of CDT1 and MDM2-dependent ubiquitination of TP53 in response to radiation-induced DNA damage and during DNA replication. DCX(ERCC8) (the CSA complex) plays a role in transcription-coupled repair (TCR). May also play a role in ubiquitination of CDKN1B/p27kip when associated with CUL4 and SKP2.<ref>PMID:12732143</ref> <ref>PMID:15448697</ref> <ref>PMID:14739464</ref> <ref>PMID:15882621</ref> <ref>PMID:16260596</ref> <ref>PMID:16482215</ref> <ref>PMID:17079684</ref> <ref>PMID:16407242</ref> <ref>PMID:16407252</ref> <ref>PMID:16678110</ref> <ref>PMID:16940174</ref> <ref>PMID:17041588</ref> <ref>PMID:16473935</ref> <ref>PMID:18593899</ref> <ref>PMID:18381890</ref> <ref>PMID:18332868</ref> [[http://www.uniprot.org/uniprot/UNG_HUMAN UNG_HUMAN]] Excises uracil residues from the DNA which can arise as a result of misincorporation of dUMP residues by DNA polymerase or due to deamination of cytosine. [[http://www.uniprot.org/uniprot/VPRBP_HUMAN VPRBP_HUMAN]] Component of the CUL4A-RBX1-DDB1-VprBP/DCAF1 E3 ubiquitin-protein ligase complex, VprBP/DCAF1 may function as the substrate recognition module within this complex. For example, VprBP/DCAF1 targets NF2 to the E3 ubiquitin-ligase complex for ubiquitination and subsequent proteasome-dependent degradation. In case of infection by HIV-1 virus, it is recruited by HIV-1 Vpr in order to hijack the CUL4A-RBX1-DDB1 function leading to arrest the cell cycle in G2 phase, and also to protect the viral protein from proteasomal degradation by another E3 ubiquitin ligase. In case of infection by HIV-2 virus, it is recruited by HIV-2 Vpx in order to hijack the CUL4A-RBX1-DDB1 function leading to enhanced efficiency of macrophage infection and promotion of the replication of cognate primate lentiviruses in cells of monocyte/macrophage lineage. Associated with chromatin in a DDB1-independent and cell cycle-dependent manner, VprBP/DCAF1 is recruited to chromatin as DNA is being replicated and is released from chromatin before mitosis.<ref>PMID:17314515</ref> <ref>PMID:17620334</ref> <ref>PMID:17626091</ref> <ref>PMID:17630831</ref> <ref>PMID:17609381</ref> <ref>PMID:17559673</ref> <ref>PMID:18524771</ref> <ref>PMID:18606781</ref> <ref>PMID:18332868</ref> <ref>PMID:18464893</ref> <ref>PMID:19264781</ref> <ref>PMID:19923175</ref> <ref>PMID:23063525</ref> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | The HIV-1 accessory protein Vpr is required for efficient viral infection of macrophages and promotion of viral replication in T cells. Vpr's biological activities are closely linked to the interaction with human DCAF1, a cellular substrate receptor of the Cullin4-RING E3 ubiquitin ligase (CRL4) of the host ubiquitin-proteasome-mediated protein degradation pathway. The molecular details of how Vpr usurps the protein degradation pathway have not been delineated. Here we present the crystal structure of the DDB1-DCAF1-HIV-1-Vpr-uracil-DNA glycosylase (UNG2) complex. The structure reveals how Vpr engages with DCAF1, creating a binding interface for UNG2 recruitment in a manner distinct from the recruitment of SAMHD1 by Vpx proteins. Vpr and Vpx use similar N-terminal and helical regions to bind the substrate receptor, whereas different regions target the specific cellular substrates. Furthermore, Vpr uses molecular mimicry of DNA by a variable loop for specific recruitment of the UNG2 substrate. | ||
| - | + | The DDB1-DCAF1-Vpr-UNG2 crystal structure reveals how HIV-1 Vpr steers human UNG2 toward destruction.,Wu Y, Zhou X, Barnes CO, DeLucia M, Cohen AE, Gronenborn AM, Ahn J, Calero G Nat Struct Mol Biol. 2016 Aug 29. doi: 10.1038/nsmb.3284. PMID:27571178<ref>PMID:27571178</ref> | |
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | [[Category: | + | </div> |
| + | <div class="pdbe-citations 5jk7" style="background-color:#fffaf0;"></div> | ||
| + | == References == | ||
| + | <references/> | ||
| + | __TOC__ | ||
| + | </StructureSection> | ||
| + | [[Category: Non-specific serine/threonine protein kinase]] | ||
| + | [[Category: Ahn, J]] | ||
| + | [[Category: Calero, G]] | ||
| + | [[Category: Wu, Y]] | ||
| + | [[Category: Cullin4-ring e3 ubiquitin ligase hiv-1 vpr ung2]] | ||
| + | [[Category: Dna binding protein-hydrolase complex]] | ||
| + | [[Category: Viral protein-dna binding protein complex]] | ||
Revision as of 21:31, 5 October 2016
The X-ray structure of the DDB1-DCAF1-Vpr-UNG2 complex
| |||||||||||