User:Wally Novak/Sandbox Miller
From Proteopedia
| Line 8: | Line 8: | ||
The remaining N-terminus domain of DNA ligase I is involved in regulating interactions with outside proteins. Proliferating cell nuclear antigen (PCNA), DNA polymerase β (POLB), and replication factor C (RFC) are known to bind in to this domain of DNA ligase I.<ref>Tomkinson, A.E.; Vijayakumar, S.; Pascal, J.M.; Ellenberger, T. ''Chem. Rev.'' '''2006''', ''106'', 687-669;Pascal, J.M.; O'Brien, P.J.; Tomkinson, A.E.; Ellenberger, T. ''Nature'' '''2004''', ''432'', 473-478.</ref> PCNA, in fact, appears to lock DNA ligase into its catalytic state thereby increasing throughput.<ref>Pascal, J.M.; Tsodikov, O.V.; Hura, G.L.; Song, W.; Cotner, E.A.; Classen, S.; Tomkinson, A.E.; Tainer, J.A.; Ellenberger, T. ''Mol. Cell'' '''2006''', ''24'', 279-291</ref> The N-terminus interactive domain also has three Ser which can be phosphorylated; the phosphorylation of these residues at various parts of the cell cycle suggest regulation by post-translational modification.<ref>Ellenberger, T.; Tomkinson, A.E. ''Annu. Rev. Biochem.'' '''2008''', ''77'', 13-38.</ref> | The remaining N-terminus domain of DNA ligase I is involved in regulating interactions with outside proteins. Proliferating cell nuclear antigen (PCNA), DNA polymerase β (POLB), and replication factor C (RFC) are known to bind in to this domain of DNA ligase I.<ref>Tomkinson, A.E.; Vijayakumar, S.; Pascal, J.M.; Ellenberger, T. ''Chem. Rev.'' '''2006''', ''106'', 687-669;Pascal, J.M.; O'Brien, P.J.; Tomkinson, A.E.; Ellenberger, T. ''Nature'' '''2004''', ''432'', 473-478.</ref> PCNA, in fact, appears to lock DNA ligase into its catalytic state thereby increasing throughput.<ref>Pascal, J.M.; Tsodikov, O.V.; Hura, G.L.; Song, W.; Cotner, E.A.; Classen, S.; Tomkinson, A.E.; Tainer, J.A.; Ellenberger, T. ''Mol. Cell'' '''2006''', ''24'', 279-291</ref> The N-terminus interactive domain also has three Ser which can be phosphorylated; the phosphorylation of these residues at various parts of the cell cycle suggest regulation by post-translational modification.<ref>Ellenberger, T.; Tomkinson, A.E. ''Annu. Rev. Biochem.'' '''2008''', ''77'', 13-38.</ref> | ||
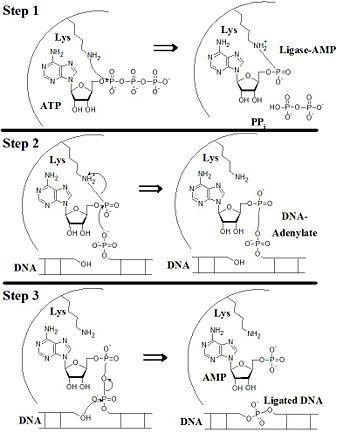
== Ligating Mechanism == | == Ligating Mechanism == | ||
| - | [[Image:Ligase Ligating Mech.jpg]] | + | [[Image:Ligase Ligating Mech.jpg|350 px]] |
Revision as of 18:27, 10 October 2016
|
DNA ligases are a group of proteins responsible for the joining DNA fragments which arise from various cellular events: ligating Okazaki fragments from replication, various repair mechanisms, and recombination. [1] The ligation proceeds via a three step process uniting the 3'-OH to the 5'-phosphate with an ATP or NAD+ cofactor. [2] DNA ligase I is a member of this family expressed throughout eukaryotes. Included is a summary of its functionality covering the conserved structural domains, ligating mechanism, and involvement in replication as well as DNA repair mechanisms.
Contents |
Structural Domains
DNA ligase I has four structural domains spread over a 919 residue monomeric protein. Three of the four domains are highlighted in the . The DNA-binding domain (DBD) is in red; the adenylation domain (AdD), green; the OB-fold domain (OBD), yellow; and the DNA, grey. The first 232 residues containing domains that interact with other regulative proteins were excluded from the source structure that Ellenberger and colleagues studied.[3]
The DBD has straightforward function, the binding of DNA, which it accomplishes using mostly polar residues with some basic, cationic ones along the minor groove of the DNA.[4] Remarkably, the stabilization of the strand opposite the nick in a partially unwound state by the DBD facilitates ligation in trans.[5] The AdD serves as the catalytic core of DNA ligase I and operates via a highly conserved Lys residue.[6] The OBD actually has multiple conformations depending on whether or not DNA is bound.[7] Upon DNA binding, the OBD - Phe 635 and 872 sit in the minor groove and perhaps π-stack while Asp 570 and Arg 871 form salt-bridges connecting the AdD and OBD.[8] Unsurprisingly, most of these DNA binding interactions appear to be facilitated by metals.
The remaining N-terminus domain of DNA ligase I is involved in regulating interactions with outside proteins. Proliferating cell nuclear antigen (PCNA), DNA polymerase β (POLB), and replication factor C (RFC) are known to bind in to this domain of DNA ligase I.[9] PCNA, in fact, appears to lock DNA ligase into its catalytic state thereby increasing throughput.[10] The N-terminus interactive domain also has three Ser which can be phosphorylated; the phosphorylation of these residues at various parts of the cell cycle suggest regulation by post-translational modification.[11]
Ligating Mechanism
Okazaki Fragments and Replication
DNA Repair
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
- ↑ Ellenberger, T.; Tomkinson, A.E. Annu. Rev. Biochem. 2008, 77, 13-38; Shuman, S. J. Biol. Chem. 2009, 284, 17365-17369.
- ↑ Ellenberger, T.; Tomkinson, A.E. Annu. Rev. Biochem. 2008, 77, 13-38; Shuman, S. J. Biol. Chem. 2009, 284, 17365-17369; Tomkinson, A.E.; Vijayakumar, S.; Pascal, J.M.; Ellenberger, T. Chem. Rev. 2006, 106, 687-669.
- ↑ Pascal, J.M.; O'Brien, P.J.; Tomkinson, A.E.; Ellenberger, T. Nature 2004, 432, 473-478.
- ↑ Pascal, J.M.; O'Brien, P.J.; Tomkinson, A.E.; Ellenberger, T. Nature 2004, 432, 473-478.
- ↑ Tomkinson, A.E.; Vijayakumar, S.; Pascal, J.M.; Ellenberger, T. Chem. Rev. 2006, 106, 687-669.
- ↑ Ellenberger, T.; Tomkinson, A.E. Annu. Rev. Biochem. 2008, 77, 13-38.
- ↑ Pascal, J.M.; O'Brien, P.J.; Tomkinson, A.E.; Ellenberger, T. Nature 2004, 432, 473-478.
- ↑ Ellenberger, T.; Tomkinson, A.E. Annu. Rev. Biochem. 2008, 77, 13-38; Pascal, J.M.; O'Brien, P.J.; Tomkinson, A.E.; Ellenberger, T. Nature 2004, 432, 473-478.
- ↑ Tomkinson, A.E.; Vijayakumar, S.; Pascal, J.M.; Ellenberger, T. Chem. Rev. 2006, 106, 687-669;Pascal, J.M.; O'Brien, P.J.; Tomkinson, A.E.; Ellenberger, T. Nature 2004, 432, 473-478.
- ↑ Pascal, J.M.; Tsodikov, O.V.; Hura, G.L.; Song, W.; Cotner, E.A.; Classen, S.; Tomkinson, A.E.; Tainer, J.A.; Ellenberger, T. Mol. Cell 2006, 24, 279-291
- ↑ Ellenberger, T.; Tomkinson, A.E. Annu. Rev. Biochem. 2008, 77, 13-38.
[1] to the rescue.