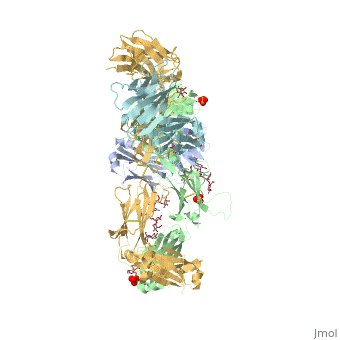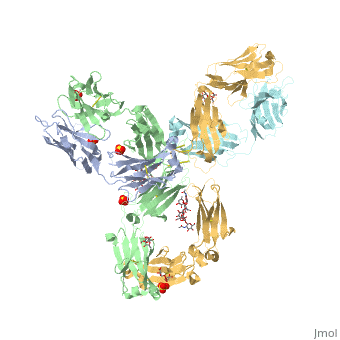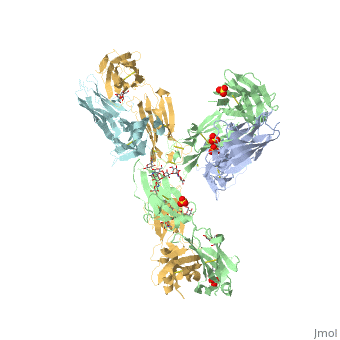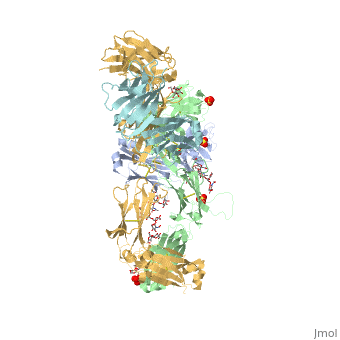We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox454
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | == Pembrolizumab == | + | ==Pembrolizumab== |
| - | < | + | <StructureSection load='5dk3' size='350' side='right' caption='Full-Length Crystal Structure of Pembrolizumab' scene=''> |
| - | + | Click above on '''edit this page''' to modify. Be careful with the < and > signs. | |
| - | + | ||
You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| Line 11: | Line 10: | ||
== Pembrolizumab/PD-1 Interaction == | == Pembrolizumab/PD-1 Interaction == | ||
| - | In order for pembrolizumab to block PD-1, pembrolizumab forms a large, flat paratope (antigen-binding site) that can sustain PD-1’s large epitope (where antibody attaches on antigen). The induced interaction between pembrolizumab and PD-1 gives rise to a surface conformational change on PD-1. The new structure of PD-1 becomes a very shallow, “crescent”-like shape, in contrast to it’s flat conformation when bound to PD-L1 <ref>DOI: 10.1038/srep35297< | + | In order for pembrolizumab to block PD-1, pembrolizumab forms a large, flat paratope (antigen-binding site) that can sustain PD-1’s large epitope (where antibody attaches on antigen). The induced interaction between pembrolizumab and PD-1 gives rise to a surface conformational change on PD-1. The new structure of PD-1 becomes a very shallow, “crescent”-like shape, in contrast to it’s flat conformation when bound to PD-L1 <ref>DOI:10.1038/srep35297</ref>. |
== PemFv/PD-1 Interaction == | == PemFv/PD-1 Interaction == | ||
| - | The Fv fragment of pembrolizumab (PemFv) can form a complex with the extracellular domain (ECD) of PD-1. Both PemFv and PD-1ECD contain interchain disulfide bonds. PemFv interacts predominantly in the major groove of PD-1, which is formed on one surface by the CC’FG antiparallel β−sheet and the BC, C’D, and FG loops. There are 15 direct hydrogen bonds between the residues, 15 water-mediated hydrogen bonds, 2 salt bridges, and many hydrophobic interactions. A very large solvent-accessible surface area of 1,137Å2 is buried on PD-1ECD due to the convoluted interaction. There are a total of 26 PD-1ECD residues involved in the interaction with PemFv, with residues in loop C’D (Pro84 to Gly90) and strand C’ (Gln75 to Lys 78) playing a major role. These key components of PD-1 mainly form interactions through salt bridges and hydrogen bonds with CRD-L3, CDR-H1, CDR-H2, CDR-H3 of pembrolizumab. It is beleived that the sugar chains of PD-1 have no phsyical contact with pembrolizumab due to the N-linked glycosylated residues (Asn49, Asn58, Asn74, and Asn116) being located away from the interaface <ref>DOI: 10.1038/srep35297< | + | The Fv fragment of pembrolizumab (PemFv) can form a complex with the extracellular domain (ECD) of PD-1. Both PemFv and PD-1ECD contain interchain disulfide bonds. PemFv interacts predominantly in the major groove of PD-1, which is formed on one surface by the CC’FG antiparallel β−sheet and the BC, C’D, and FG loops. There are 15 direct hydrogen bonds between the residues, 15 water-mediated hydrogen bonds, 2 salt bridges, and many hydrophobic interactions. A very large solvent-accessible surface area of 1,137Å2 is buried on PD-1ECD due to the convoluted interaction. There are a total of 26 PD-1ECD residues involved in the interaction with PemFv, with residues in loop C’D (Pro84 to Gly90) and strand C’ (Gln75 to Lys 78) playing a major role. These key components of PD-1 mainly form interactions through salt bridges and hydrogen bonds with CRD-L3, CDR-H1, CDR-H2, CDR-H3 of pembrolizumab. It is beleived that the sugar chains of PD-1 have no phsyical contact with pembrolizumab due to the N-linked glycosylated residues (Asn49, Asn58, Asn74, and Asn116) being located away from the interaface <ref>DOI: 10.1038/srep35297</ref>. |
== PD-L1/PD-1 Interaction == | == PD-L1/PD-1 Interaction == | ||
| - | The complex formed when protein-derived ligand, PD-L1, interacts with the inhibitory receptor, PD-1, suppresses immune responses again autoantigens and helps in peripheral immune tolerance. However, when tumors overexpress PD-L1, the interaction with PD-1 inhibits T-lymphocyte proliferation, release of cytokines, and cytotoxicity, exhausting tumor-specific T-cells. There are a total of 12 PD-1ECD residues that are involved in forming the complex with the N-terminal half of PD-L1ECD (PD-L1ECD-N). Nine hydrogen bonds, 3 water-mediated hydrogen bonds, 2 salt bridges, and numerous hydrophobic interactions make up the PD-1ECD/PD-L1ECD-N interaction.The CC’FG sheet within both proteins is the main interaction point. A hydrophobic surface patch is formed when the PD-1ECD is in complex with PD-L1ECD-N. The PD-1ECD residues involved include Val64, Tyr68, Ile126, Leu128, Ala132 and Ile134. Numerous hydrophilic amino acids that encircle PD-L1ECD-N form salt bridges and hydrogen bonds with Asn66, Tyr68, Gln75, Thr76, Asp77, Lys78, Ala132 and Glu136 of PD-1ECD <ref>DOI: 10.1038/srep35297< | + | The complex formed when protein-derived ligand, PD-L1, interacts with the inhibitory receptor, PD-1, suppresses immune responses again autoantigens and helps in peripheral immune tolerance. However, when tumors overexpress PD-L1, the interaction with PD-1 inhibits T-lymphocyte proliferation, release of cytokines, and cytotoxicity, exhausting tumor-specific T-cells. There are a total of 12 PD-1ECD residues that are involved in forming the complex with the N-terminal half of PD-L1ECD (PD-L1ECD-N). Nine hydrogen bonds, 3 water-mediated hydrogen bonds, 2 salt bridges, and numerous hydrophobic interactions make up the PD-1ECD/PD-L1ECD-N interaction.The CC’FG sheet within both proteins is the main interaction point. A hydrophobic surface patch is formed when the PD-1ECD is in complex with PD-L1ECD-N. The PD-1ECD residues involved include Val64, Tyr68, Ile126, Leu128, Ala132 and Ile134. Numerous hydrophilic amino acids that encircle PD-L1ECD-N form salt bridges and hydrogen bonds with Asn66, Tyr68, Gln75, Thr76, Asp77, Lys78, Ala132 and Glu136 of PD-1ECD <ref>DOI: 10.1038/srep35297</ref>. |
== Mechanism == | == Mechanism == | ||
| - | Pembrolizumab works as a PD-1 pathway inhibitor. As an inhibitor it targets the cell death of PD-1 and blocks the immune checkpoint pathway. PD-1 is expressed on the surface of t-cells. T-cells are main components of the immune response in the body. The main ligands that interact with this receptor are PD-L1 and PD-L2, which are expressed by some tumors and inhibit t-cell function when bound to PD-1 | + | Pembrolizumab works as a PD-1 pathway inhibitor. As an inhibitor it targets the cell death of PD-1 and blocks the immune checkpoint pathway. PD-1 is expressed on the surface of t-cells. T-cells are main components of the immune response in the body. The main ligands that interact with this receptor are PD-L1 and PD-L2, which are expressed by some tumors and inhibit t-cell function when bound to PD-1 <ref>doi 10.1007/s40265-016-0543-x</ref>. Pembrolizumab has a very high affinity to PD-1, allowing it to block the interaction between PD-1 with PD-L1 and PD-L2. It antagonizes the interaction between PD-1 and its known ligands, re-activating anti-tumor immunity <ref>doi 10.1080/17425255.2016.1216976</ref>. The PD-1/PD-L1 interaction inhibits t-lymphocyte proliferation, releases cytokines and cytotoxicity, and exhausts tumor-specific t-cells. The inhibition of this pathway reverses the exhausted t-cell phenotype and normalizes the anti-tumor response. Pembrolizumab may cause inflammatory side effects <ref>DOI: 10.1038/srep35297</ref>. |
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
Revision as of 04:44, 15 November 2016
Pembrolizumab
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol. 2016 Oct;12(10):1247-53. doi:, 10.1080/17425255.2016.1216976. Epub 2016 Aug 16. PMID:27485741 doi:http://dx.doi.org/10.1080/17425255.2016.1216976
- ↑ Horita S, Nomura Y, Sato Y, Shimamura T, Iwata S, Nomura N. High-resolution crystal structure of the therapeutic antibody pembrolizumab bound to the human PD-1. Sci Rep. 2016 Oct 13;6:35297. doi: 10.1038/srep35297. PMID:27734966 doi:http://dx.doi.org/10.1038/srep35297
- ↑ Horita S, Nomura Y, Sato Y, Shimamura T, Iwata S, Nomura N. High-resolution crystal structure of the therapeutic antibody pembrolizumab bound to the human PD-1. Sci Rep. 2016 Oct 13;6:35297. doi: 10.1038/srep35297. PMID:27734966 doi:http://dx.doi.org/10.1038/srep35297
- ↑ Horita S, Nomura Y, Sato Y, Shimamura T, Iwata S, Nomura N. High-resolution crystal structure of the therapeutic antibody pembrolizumab bound to the human PD-1. Sci Rep. 2016 Oct 13;6:35297. doi: 10.1038/srep35297. PMID:27734966 doi:http://dx.doi.org/10.1038/srep35297
- ↑ Deeks ED. Pembrolizumab: A Review in Advanced Melanoma. Drugs. 2016 Mar;76(3):375-86. doi: 10.1007/s40265-016-0543-x. PMID:26846323 doi:http://dx.doi.org/10.1007/s40265-016-0543-x
- ↑ Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol. 2016 Oct;12(10):1247-53. doi:, 10.1080/17425255.2016.1216976. Epub 2016 Aug 16. PMID:27485741 doi:http://dx.doi.org/10.1080/17425255.2016.1216976
- ↑ Horita S, Nomura Y, Sato Y, Shimamura T, Iwata S, Nomura N. High-resolution crystal structure of the therapeutic antibody pembrolizumab bound to the human PD-1. Sci Rep. 2016 Oct 13;6:35297. doi: 10.1038/srep35297. PMID:27734966 doi:http://dx.doi.org/10.1038/srep35297