We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ribavirin
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
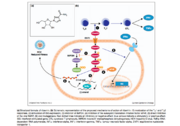
Immunomodulation by Ribavirin may be responsible for the drug’s antiviral properties. It has been suggested that the natural CD4+ helper T cell response may be altered in the presence of Ribavirin. It is thought that ribavirin may enhance the T helper 1 response, resulting in greater clearance of virus <ref>doi: 10.1111/j.1478-3231.2008.01896.x</ref>. However, there is conflicting evidence suggesting that the T helper 2 response may be implicated in this process instead <ref>doi: 10.1111/j.1440-1746.2008.05320.x</ref>. Another possible mechanism involves the enhancement of interferon-stimulated gene (ISG) expression by Ribavirin. When a cell becomes infected with a virus, it may release interferons. Interferons are signaling molecules that function in a paracrine fashion to induce an antiviral state in neighboring cells, protecting them from infection. Ribavirin is thought to enhance the interferon signaling pathway <ref>doi: 10.1002/hep.21853</ref>, resulting in a wider antiviral response. This theory has been supported in studies using cell culture models <ref>doi: 10.1002/hep.23985</ref>. It has also been suggested that the relationship between Ribavirin and inosine 5’-monophosphate dehydrogenase (IMPDH) may impact this process. IMPDH play a significant role in the guanine nucleotide synthesis pathway. It results in the conversion of inosine 5’-monophosphate to xanthine 5’-monophosphate, which is an intermediate for the nucleotide guanosine <ref>doi: 10.1002/chin.200823265</ref>. Therefore, modulation of IMPDH activity affects a cell’s reservoir of guanosine. Ribavirin has been shown to function as a competitive inhibitor for IMPDH <ref>PMID: 4197928</ref>. Because guanosine triphosphate (GTP) plays a critical role in the viral genome replication process, inhibition of IMPDH would result in the prevention of viral replication. | Immunomodulation by Ribavirin may be responsible for the drug’s antiviral properties. It has been suggested that the natural CD4+ helper T cell response may be altered in the presence of Ribavirin. It is thought that ribavirin may enhance the T helper 1 response, resulting in greater clearance of virus <ref>doi: 10.1111/j.1478-3231.2008.01896.x</ref>. However, there is conflicting evidence suggesting that the T helper 2 response may be implicated in this process instead <ref>doi: 10.1111/j.1440-1746.2008.05320.x</ref>. Another possible mechanism involves the enhancement of interferon-stimulated gene (ISG) expression by Ribavirin. When a cell becomes infected with a virus, it may release interferons. Interferons are signaling molecules that function in a paracrine fashion to induce an antiviral state in neighboring cells, protecting them from infection. Ribavirin is thought to enhance the interferon signaling pathway <ref>doi: 10.1002/hep.21853</ref>, resulting in a wider antiviral response. This theory has been supported in studies using cell culture models <ref>doi: 10.1002/hep.23985</ref>. It has also been suggested that the relationship between Ribavirin and inosine 5’-monophosphate dehydrogenase (IMPDH) may impact this process. IMPDH play a significant role in the guanine nucleotide synthesis pathway. It results in the conversion of inosine 5’-monophosphate to xanthine 5’-monophosphate, which is an intermediate for the nucleotide guanosine <ref>doi: 10.1002/chin.200823265</ref>. Therefore, modulation of IMPDH activity affects a cell’s reservoir of guanosine. Ribavirin has been shown to function as a competitive inhibitor for IMPDH <ref>PMID: 4197928</ref>. Because guanosine triphosphate (GTP) plays a critical role in the viral genome replication process, inhibition of IMPDH would result in the prevention of viral replication. | ||
| - | Ribavirin has also been shown to act as an inhibitor for eIF4E, a protein of the translation initiation complex <ref>doi: 10.1073/pnas.0406927102</ref> | + | Ribavirin has also been shown to act as an inhibitor for eIF4E, a protein of the translation initiation complex <ref>doi: 10.1073/pnas.0406927102</ref>. Because of its structural similarity to guanosine, it mimics the 7-methyl guanosine mRNA cap, preventing translation. This would result in reduced capacity for viral replication within an infected cell. The Hepatitis C viral genome is replicated by RNA-dependent RNA polymerase (RdRp). A modified form of Ribavirin, Ribavirin 5’-triphosphate (RTP) is also believed to directly inhibit RdRp activity <ref>doi: 10.1002/rmv.483</ref>, resulting in lower rates of genome replication. If Ribavirin is converted to the monophosphate form, RMP, it is believed to be incorporated into the viral genome, functioning as a mutagen. |
Revision as of 04:25, 16 November 2016
1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide [1]
| |||||||||||
References
- Gish, R. G. Treating HCV with ribavirin analogue and ribavirin-like molecules. Journal of Antimicrobial Chemotherapy. 2005, November 17;1-6. doi:10.1093/jac/dki405
- Chung, R.T., Gale, M.J., Polyak, S.J., Lemon, S.M., Liang, T.J., & Hoofnagle, J.H. Mechanisms of action of interferon and ribavirin in chronic hepatitis C: Summary of a workshop. Hepatology. 2008;47 (1), 306-320. doi: 10.1002/hep.22070
- Paeshuyse, J, Dallmeier, K, Neyts, J. Ribavirin for the treatment of chronic hepatitis C virus infection: a review of the proposed mechanisms of action. Current Opinion in Virology. 2011;1(6) 590-598. doi: 10.1016/j.coviro.2011.10.030
- National Heart, Lung and Blood Institute. Pneumonia. 2016, September 26; Retrieved from https://www.nhlbi.nih.gov/health/health-topics/topics/pnu
- Foster, G. Pegylated interferons for the treatment of chronic Hepatitis C. Drugs. 2010;70(2):147-165. doi:10.2165/11531990-000000000-00000
- Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 2008, 28:1332-1343. doi: 10.1111/j.1478-3231.2008.01896.x
- Fujimoto T, Tomimatsu M, Iga D, Endo H, Otsuka K. Changes in the Th1/Th2 ratio during a 24-week course of an interferon alpha-2b plus ribavirin combination therapy for patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2008, 23:E432- E437. doi: 10.1111/j.1440-1746.2008.05320.x
- Feld, JJ, Nanda, S, Huang, Y, Chen, W, Cam, M, Pusek, SN, Schwigler, LM, Theodore, D, Zacks, SL, Liang, TJ, Fried, MW. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecule pathways for treatment response. Hepatol. 2007, 46(5): 1548-1563. doi: 10.1002/hep.21853
- Thomas E, Feld JJ, Li QS, Hu ZY, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon stimulated gene induction in hepatitis C virus cell culture models. Hepatology 2011, 53:32-41. doi: 10.1002/hep.23985
- Shu QN, Nair V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med. Res. Rev. 2008, 28:219-232. doi: 10.1002/chin.200823265
- Streeter, DG, Witkowski, JT, Khare, GP, Sidwell, RW, Bauer, RJ, Robins, RK, Simon, LN. Mechanisms of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole) a new broad spectrum antiviral agent. Proc. Natl. Acad. Sci. 1973, 70:1174-1178. PMID: 4197928
- Kentsis, A, Topisirovic, I, Culjkovic, B, Shao, L, Borden, KLB. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the guanosine mRNA cap. Proc. Natl. Acad. Sci. 2004, 101:18105-18110. Doi: 10.1073/pnas.0406927102
- Graci, JD, Cameron, CE. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16: 37-48. Doi: 10.1002/rmv.483
Proteopedia Page Contributors and Editors (what is this?)
Jamie Costa, Edmond R Atalla, Katherine Reynolds, Taylor H. Derby, Shannon Shaughnessy, Alexander Berchansky