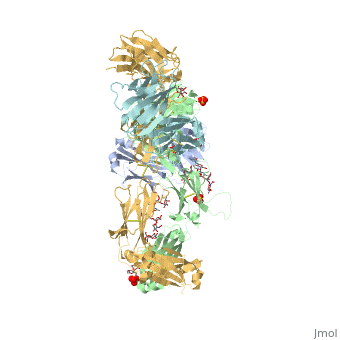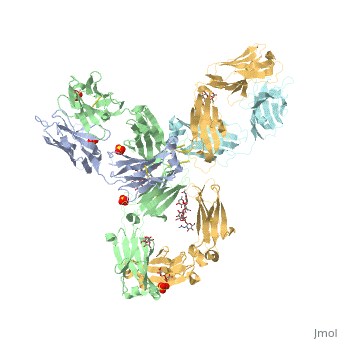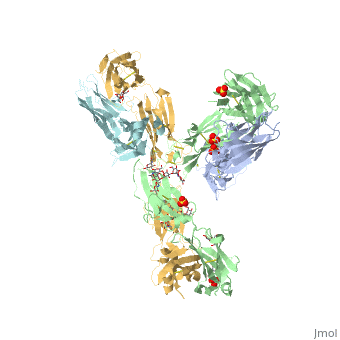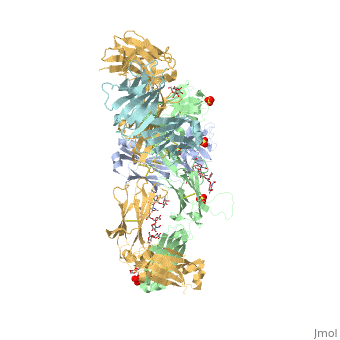This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox454
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structure and Function == | == Structure and Function == | ||
| - | Pembrolizumab, or Keytruda, is an immunoglobulin G4 (IgG4)-kappa humanized monoclonal antibody against the programmed cell death-1 (PD-1) receptor. It is a very compact molecule with an asymmetrical Y-shape. The short compact hinge region inflicts constraints on the molecule that creates the abnormal crystallizable tail region (Fc domain) compared to other immunoglobulin G (IgG) proteins. The Fc domain is <scene name='74/745945/Glycosylation/1'>glycosylated</scene> at both CH2 domains on each chain and one of them is distinctively rotated 120° compared to other similar structures, making the glycan chain more solvent accessible and facing the solvent. IgG4s have a unique function where they form dynamic bispecific antibodies by exchanging half-molecules (one heavy chain/light chain pair) among themselves, called Fab-arm exchange. This makes the molecule particularly unstable and unpredictable as a treatment, but can be conquered by introducing a serine-to-proline mutation at <scene name='74/745945/Pro228/1'>amino acid 228</scene>, which prevents Fab-arm exchange and stabilizes the molecule <ref>DOI:10.1080/17425255.2016.1216976</ref>. Pembrolizumab contains an Fv fragment (PemFv) and a Fab fragment (PemFab). The Fv fragment is the variable region of the molecule where binding occurs whereas the Fab fragment constitutes the whole molecule. | + | Pembrolizumab, or Keytruda, is an immunoglobulin G4 (IgG4)-kappa humanized monoclonal antibody against the programmed cell death-1 (PD-1) receptor. It is a very compact molecule with an asymmetrical Y-shape. The short compact hinge region inflicts constraints on the molecule that creates the abnormal crystallizable tail region (Fc domain) compared to other immunoglobulin G (IgG) proteins. The Fc domain is <scene name='74/745945/Glycosylation/1'>glycosylated</scene> at both CH2 domains on each chain and one of them is distinctively rotated 120° compared to other similar structures, making the glycan chain more solvent accessible and facing the solvent. IgG4s have a unique function where they form dynamic bispecific antibodies by exchanging half-molecules (one heavy chain/light chain pair) among themselves, called Fab-arm exchange. This makes the molecule particularly unstable and unpredictable as a treatment, but can be conquered by introducing a serine-to-proline mutation at <scene name='74/745945/Pro228/1'>amino acid 228</scene>, which prevents Fab-arm exchange and stabilizes the molecule <ref name="log">DOI:10.1080/17425255.2016.1216976</ref>. Pembrolizumab contains an Fv fragment (PemFv) and a Fab fragment (PemFab). The Fv fragment is the variable region of the molecule where binding occurs whereas the Fab fragment constitutes the whole molecule. |
== Pembrolizumab/PD-1 Interaction == | == Pembrolizumab/PD-1 Interaction == | ||
| Line 22: | Line 22: | ||
== Mechanism == | == Mechanism == | ||
| - | T-cells are a major component of the immune response in the human body. T-cells have the ability to recognize cancer-related antigens as nonself and eliminate those cells <ref>doi 10.2147/DDDT.S78036</ref>. PD-L1 and PD-L2 are ligands expressed by some tumors and inhibit t-cell function when bound to PD-1, which is located on the surface of antigen-specific t-cells <ref>doi 10.1007/s40265-016-0543-x</ref>. When PD-L1 is ligated to PD-1 an adaptive immune response is produced, and this allows cancer cells to bypass the immune surveillance and grow uncontrollably. Pembrolizumab is an FDA-approved treatment that works as a PD-1 pathway inhibitor to fight metastatic melanoma, a form of cancer. As an inhibitor, Pembrolizumab targets the cell death of PD-1 and blocks the immune checkpoint pathway. Pembrolizumab has a very high affinity to PD-1, allowing it to block the interaction between PD-1 with PD-L1 and PD-L2 very efficiently. It antagonizes the interaction between PD-1 and its known ligands, and re-activates anti-tumor immunity <ref | + | T-cells are a major component of the immune response in the human body. T-cells have the ability to recognize cancer-related antigens as nonself and eliminate those cells <ref>doi 10.2147/DDDT.S78036</ref>. PD-L1 and PD-L2 are ligands expressed by some tumors and inhibit t-cell function when bound to PD-1, which is located on the surface of antigen-specific t-cells <ref>doi 10.1007/s40265-016-0543-x</ref>. When PD-L1 is ligated to PD-1 an adaptive immune response is produced, and this allows cancer cells to bypass the immune surveillance and grow uncontrollably. Pembrolizumab is an FDA-approved treatment that works as a PD-1 pathway inhibitor to fight metastatic melanoma, a form of cancer. As an inhibitor, Pembrolizumab targets the cell death of PD-1 and blocks the immune checkpoint pathway. Pembrolizumab has a very high affinity to PD-1, allowing it to block the interaction between PD-1 with PD-L1 and PD-L2 very efficiently. It antagonizes the interaction between PD-1 and its known ligands, and re-activates anti-tumor immunity <ref name="log" />. The PD-1/PD-L1 interaction inhibits t-lymphocyte proliferation, releases cytokines and cytotoxicity, and exhausts tumor-specific t-cells. The inhibition of this pathway reverses the exhausted t-cell phenotype and normalizes the anti-tumor response. Pembrolizumab may cause inflammatory side effects <ref name="horita" />. |
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
Revision as of 22:49, 16 November 2016
Pembrolizumab
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol. 2016 Oct;12(10):1247-53. doi:, 10.1080/17425255.2016.1216976. Epub 2016 Aug 16. PMID:27485741 doi:http://dx.doi.org/10.1080/17425255.2016.1216976
- ↑ 4.0 4.1 4.2 4.3 Horita S, Nomura Y, Sato Y, Shimamura T, Iwata S, Nomura N. High-resolution crystal structure of the therapeutic antibody pembrolizumab bound to the human PD-1. Sci Rep. 2016 Oct 13;6:35297. doi: 10.1038/srep35297. PMID:27734966 doi:http://dx.doi.org/10.1038/srep35297
- ↑ Rajakulendran T, Adam DN. Spotlight on pembrolizumab in the treatment of advanced melanoma. Drug Des Devel Ther. 2015 Jun 4;9:2883-6. doi: 10.2147/DDDT.S78036. eCollection, 2015. PMID:26082618 doi:http://dx.doi.org/10.2147/DDDT.S78036
- ↑ Deeks ED. Pembrolizumab: A Review in Advanced Melanoma. Drugs. 2016 Mar;76(3):375-86. doi: 10.1007/s40265-016-0543-x. PMID:26846323 doi:http://dx.doi.org/10.1007/s40265-016-0543-x