We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
CRISPR-Cas
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
The CRISPR-Cas systems provide protection against mobile genetic elements (MGEs) — in particular, viruses and plasmids— by sequence-specific targeting of foreign DNA or RNA <ref name="Rev36">PMID:17379808</ref>. A CRISPR-cas locus generally consists of an operon of CRISPR-associated (cas) genes and a CRISPR array composed of a series of direct repeats interspaced by variable DNA sequences (known as spacers) (Fig. 1). The repeat sequences and lengths as well as the number of repeats in CRISPR arrays vary broadly, but all arrays possess the characteristic arrangement of alternating repeat and spacer sequences. The spacers are key elements of adaptive immunity, as they store the “memory” of an organism’s encounters with specific MGEs acquired as a result of a previous unsuccessful infection. This memory enables the recognition and neutralization of the invaders upon subsequent infections <ref name="Rev36">PMID:17379808</ref><ref name="Rev4">doi:10.1126/science.aad5147</ref>. | The CRISPR-Cas systems provide protection against mobile genetic elements (MGEs) — in particular, viruses and plasmids— by sequence-specific targeting of foreign DNA or RNA <ref name="Rev36">PMID:17379808</ref>. A CRISPR-cas locus generally consists of an operon of CRISPR-associated (cas) genes and a CRISPR array composed of a series of direct repeats interspaced by variable DNA sequences (known as spacers) (Fig. 1). The repeat sequences and lengths as well as the number of repeats in CRISPR arrays vary broadly, but all arrays possess the characteristic arrangement of alternating repeat and spacer sequences. The spacers are key elements of adaptive immunity, as they store the “memory” of an organism’s encounters with specific MGEs acquired as a result of a previous unsuccessful infection. This memory enables the recognition and neutralization of the invaders upon subsequent infections <ref name="Rev36">PMID:17379808</ref><ref name="Rev4">doi:10.1126/science.aad5147</ref>. | ||
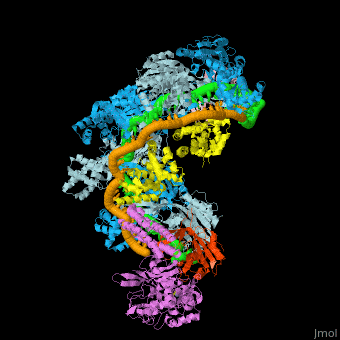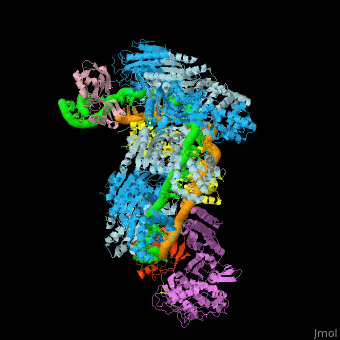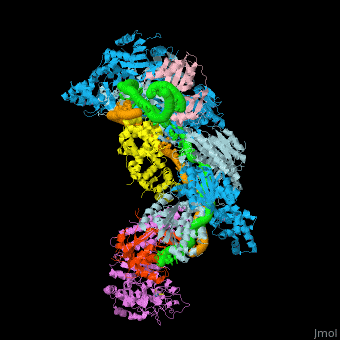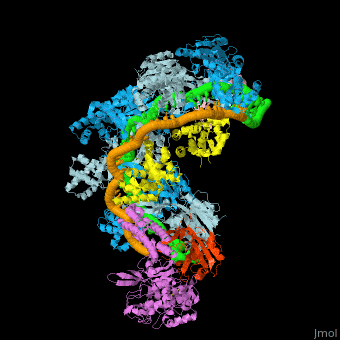
| - | + | [[Image:F1r.jpg|left|450px|thumb|Fig. 1 Overview of the CRISPR-Cas systems. (A) Architecture of class 1 (multiprotein effector complexes) and class 2 (single-protein effector complexes) CRISPR-Cas systems. (B) CRISPR-Cas adaptive immunity is mediated by CRISPR RNAs (crRNAs) and Cas proteins, which form multicomponent CRISPR ribonucleoprotein (crRNP) complexes. The first stage is adaptation, which occurs upon entry of an invading mobile genetic element (in this case, a viral genome). Cas1 (blue) and Cas2 (yellow) proteins select and process the invading DNA, and thereafter, a protospacer (orange) is integrated as a new spacer at the leader end of the CRISPR array [repeat sequences (gray) that separate similar-sized, invader-derived spacers (multiple colors)]. During the second stage, expression, the CRISPR locus is transcribed and the pre-crRNA is processed into mature crRNA guides by Cas (e.g., Cas6) or non-Cas proteins (e.g., RNase III). During the final interference stage, the Cas-crRNA complex scans invading DNA for a complementary nucleic acid target, after which the target is degraded by a Cas nuclease. From <ref name="Rev4">doi:10.1126/science.aad5147</ref>]] | |
| - | Most prokaryotes employ an RNA-based adaptive immune system known as CRISPR–Cas to identify and eliminate genetic parasites.<ref name="Rev3">PMID:25468820</ref> Upon detecting viral or plasmid DNA in the cell, bacteria and archaea with active CRISPR systems respond by integrating short fragments of foreign DNA into the host chromosome at one end of the CRISPR locus (Figure 1) <ref name="Rev36">PMID:17379808</ref><ref name="Rev37">doi:10.1038/nature09523</ref><ref name="Rev38">doi:10.1111/mmi.12640</ref>. Such loci serve as molecular vaccination cards by maintaining a genetic record of prior encounters with foreign transgressors. The defining feature of CRISPR loci is a series of direct repeats (~20–50 bp) separated by unique spacer sequences of a similar length (Figure 1) <ref name="Rev39">doi:10.1186/1745-6150-1-7</ref><ref name="Rev310">doi:10.1186/gb-2007-8-4-r61</ref>. Following transcription, CRISPR sequences are processed into short CRISPR-derived RNAs (crRNAs) <ref name="Rev312">doi:10.1126/science.1159689</ref>: | + | ???Most prokaryotes employ an RNA-based adaptive immune system known as CRISPR–Cas to identify and eliminate genetic parasites.<ref name="Rev3">PMID:25468820</ref> Upon detecting viral or plasmid DNA in the cell, bacteria and archaea with active CRISPR systems respond by integrating short fragments of foreign DNA into the host chromosome at one end of the CRISPR locus (Figure 1) <ref name="Rev36">PMID:17379808</ref><ref name="Rev37">doi:10.1038/nature09523</ref><ref name="Rev38">doi:10.1111/mmi.12640</ref>. Such loci serve as molecular vaccination cards by maintaining a genetic record of prior encounters with foreign transgressors. The defining feature of CRISPR loci is a series of direct repeats (~20–50 bp) separated by unique spacer sequences of a similar length (Figure 1) <ref name="Rev39">doi:10.1186/1745-6150-1-7</ref><ref name="Rev310">doi:10.1186/gb-2007-8-4-r61</ref>. Following transcription, CRISPR sequences are processed into short CRISPR-derived RNAs (crRNAs) <ref name="Rev312">doi:10.1126/science.1159689</ref>: |
''Examples of 3D structures of CRISPR RNA (crRNA)'' | ''Examples of 3D structures of CRISPR RNA (crRNA)'' | ||
| Line 35: | Line 35: | ||
The <scene name='74/742625/Cv3/4'>optimal DNA target of the complex is determined by a Watson–Crick base pairing of a short ∼20-nt sequence within sgRNA (within the crRNA in wild-type)</scene>, termed the guide sequence, adjacent to a <scene name='74/742625/Cv3/10'>few nucleotide long conserved motif recognized directly by Cas9 protein (protospacer adjacent motif, PAM)</scene> <ref name="Jinek">PMID:22745249</ref><ref name="Prin6">PMID:22949671</ref>. Despite this, a <scene name='74/742625/Cv/44'>few mismatches between guide sequence and target DNA can be tolerated</scene> <ref name="Jinek">PMID:22745249</ref><ref name="Prin7">PMID:23452860</ref><ref name="Prin8">PMID:23761437</ref><ref name="Prin9">PMID:24837660</ref>, more so within the 5’ proximal position of the guide sequence. | The <scene name='74/742625/Cv3/4'>optimal DNA target of the complex is determined by a Watson–Crick base pairing of a short ∼20-nt sequence within sgRNA (within the crRNA in wild-type)</scene>, termed the guide sequence, adjacent to a <scene name='74/742625/Cv3/10'>few nucleotide long conserved motif recognized directly by Cas9 protein (protospacer adjacent motif, PAM)</scene> <ref name="Jinek">PMID:22745249</ref><ref name="Prin6">PMID:22949671</ref>. Despite this, a <scene name='74/742625/Cv/44'>few mismatches between guide sequence and target DNA can be tolerated</scene> <ref name="Jinek">PMID:22745249</ref><ref name="Prin7">PMID:23452860</ref><ref name="Prin8">PMID:23761437</ref><ref name="Prin9">PMID:24837660</ref>, more so within the 5’ proximal position of the guide sequence. | ||
| - | An interference complex of CRISPR-associated (Cas) proteins uses the mature crRNA as a guide to target and destroy foreign nucleic acids bearing sequence complementarity <ref name="Rev37">doi:10.1038/nature09523</ref><ref name="Rev312">doi:10.1126/science.1159689</ref>. In addition to providing adaptive immunity, the CRISPR pathway can also play a role in endogenous gene regulation. CRISPR loci are flanked by a diverse set of ''cas'' genes that define three major CRISPR types (Types I–III) based on gene conservation and locus organization. The ''cas6'' gene family encodes a set of RNA endonucleases responsible for crRNA processing in Type I and Type III CRISPR systems. Type II systems use a trans-activating RNA (tracrRNA) together with endogenous RNase III for crRNA maturation (Figure 1). Despite minimal sequence homology, Cas6s have several conserved structural features that facilitate binding of both the pre-crRNA and their crRNA product with high affinity. In most CRISPR systems, due to the pseudo-palindromic nature of the repeat sequence,the pre-crRNA adopts a stem loop structure that is bound sequence- and shape-specifically and cleaved at its base.<ref name="Rev3">PMID:25468820</ref> For example, PaeCas6f (Csy4) from ''Pseudomonas aeruginosa'' ([[2xli]]) <scene name='74/742625/Cv4/20'>binds selectively and cleaves pre-crRNAs using phylogenetically conserved serine and histidine residues</scene> in the active site. Some pre-crRNAs are predicted to be unstructured in solution and thus may be bound differently, although base pairing may be stabilized by protein interactions <ref name="Rev310">doi:10.1186/gb-2007-8-4-r61</ref>. In Type I-B, I-C, I-E, and I-F systems, the endoRNase stays bound to the crRNA and assembles into a complex with other Cas proteins for downstream targeting <ref name="Rev312">doi:10.1126/science.1159689</ref>, while in Type I-A and III systems, the crRNA alone is loaded into the targeting complex and Cas6 dissociates <ref name="Rev3">PMID:25468820</ref> | + | An interference complex of CRISPR-associated (Cas) proteins uses the mature crRNA as a guide to target and destroy foreign nucleic acids bearing sequence complementarity <ref name="Rev37">doi:10.1038/nature09523</ref><ref name="Rev312">doi:10.1126/science.1159689</ref>. In addition to providing adaptive immunity, the CRISPR pathway can also play a role in endogenous gene regulation. CRISPR loci are flanked by a diverse set of ''cas'' genes that define three major CRISPR types (Types I–III) based on gene conservation and locus organization. The ''cas6'' gene family encodes a set of RNA endonucleases responsible for crRNA processing in Type I and Type III CRISPR systems. Type II systems use a trans-activating RNA (tracrRNA) together with endogenous RNase III for crRNA maturation (Figure 1). Despite minimal sequence homology, Cas6s have several conserved structural features that facilitate binding of both the pre-crRNA and their crRNA product with high affinity. In most CRISPR systems, due to the pseudo-palindromic nature of the repeat sequence,the pre-crRNA adopts a stem loop structure that is bound sequence- and shape-specifically and cleaved at its base.<ref name="Rev3">PMID:25468820</ref> For example, PaeCas6f (Csy4) from ''Pseudomonas aeruginosa'' ([[2xli]]) <scene name='74/742625/Cv4/20'>binds selectively and cleaves pre-crRNAs using phylogenetically conserved serine and histidine residues</scene> in the active site. Some pre-crRNAs are predicted to be unstructured in solution and thus may be bound differently, although base pairing may be stabilized by protein interactions <ref name="Rev310">doi:10.1186/gb-2007-8-4-r61</ref>. In Type I-B, I-C, I-E, and I-F systems, the endoRNase stays bound to the crRNA and assembles into a complex with other Cas proteins for downstream targeting <ref name="Rev312">doi:10.1126/science.1159689</ref>, while in Type I-A and III systems, the crRNA alone is loaded into the targeting complex and Cas6 dissociates <ref name="Rev3">PMID:25468820</ref>. The Type I interference complex is known as '''Cascade''' ('''C'''RISPR-'''as'''sociated '''c'''omplex for '''a'''ntiviral '''de'''fense), the CRISPR subtype from which it derives is denoted with a slash (e.g., the Cascade complex from a Type I-E CRISPR system is known as Cascade/I-E). Type III-A and III-B systems use the Csm and Cmr complex, respectively (Figure 1)<ref name="Rev3">PMID:25468820</ref>. |
=Summary of the most extensively characterized CRISPR endoribonucleases<ref name="Rev3">PMID:25468820</ref><ref name="Rev4">doi:10.1126/science.aad5147</ref>= | =Summary of the most extensively characterized CRISPR endoribonucleases<ref name="Rev3">PMID:25468820</ref><ref name="Rev4">doi:10.1126/science.aad5147</ref>= | ||
| - | [[Image:CRISPR.jpg|left|450px|thumb|Figure 1. Overview of CRISPR RNA (crRNA) processing and comparison between CRISPR–Cas interference systems. There are three main pathways of CRISPR adaptive immunity (Types I–III) and several subtypes, each typified by a different set of Cas proteins. The first stage of the CRISPR–Cas system is acquisition, in which a foreign DNA sequence is incorporated into the host CRISPR locus. Next, the entire repeat-spacer array is transcribed into a long precursor crRNA (pre-crRNA). A single cleavage within each repeat sequence generates shorter, mature crRNAs. Some crRNAs undergo an additional trimming step. The enzymes responsible for catalysis and exact mode of crRNA processing differ in each system. The crRNA is loaded into an interference complex where it serves as a guide for targeting invasive DNA, or in Type III-B systems, RNA. N- and C-terminal RRM folds are colored teal and gray, respectively. Abbreviations: CRISPR, clustered regularly interspaced short palindromic repeat; Cas, CRISPR-associated; R, repeat; S, spacer; RRM, RNA recognition motif. From <ref name="Rev3">PMID:25468820</ref>]] | ||
| - | {{Clear}} | ||
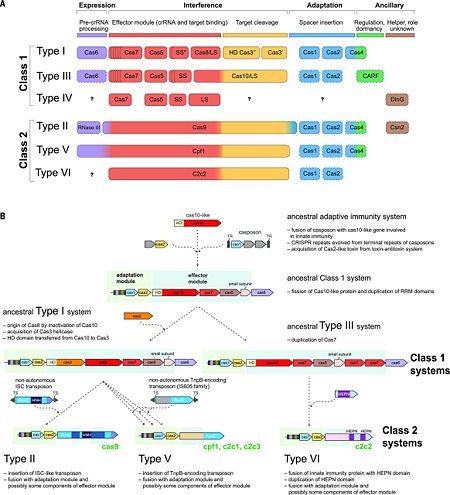
[[Image:F2r.jpg|left|450px|thumb|Figure. 2. CRISPR diversity and evolution. (A) Modular organization of the CRISPR-Cas systems. LS, large subunit; SS, small subunit. A putative small subunit that might be fused to the large subunit in several type I subtypes is indicated by an asterisk. Cas3 is shown as fusion of two distinct genes encoding the helicase Cas3′ and the nuclease HD Cas3′′; in some type I systems, these domains are encoded by separate genes. Functionally dispensable components are indicated by dashed outlines. Cas6 is shown with a thin solid outline for type I because it is dispensable in some systems, and by a dashed line for type III because most systems lack this gene and use the Cas6 provided in trans by other CRISPR-Cas loci. The two colors for Cas4 and C2c2 and three colors for Cas9 and Cpf1 reflect the contributions of these proteins to different stages of the CRISPR-Cas response (see text). The question marks indicate currently unknown components. From <ref name="Rev4">doi:10.1126/science.aad5147</ref><ref name="Rev430">doi:10.1038/nrmicro3569</ref> (B) Evolutionary scenario for the CRISPR-Cas systems. TR, terminal repeats; TS, terminal sequences; HD, HD-family endonuclease; HNH, HNH-family endonuclease; RuvC, RuvC-family endonuclease; HEPN, putative endoribonuclease of HEPN superfamily. Genes and portions of genes shown in gray denote sequences that are thought to have been encoded in the respective mobile elements but were eliminated in the course of evolution of CRISPR-Cas systems. From <ref name="Rev4">doi:10.1126/science.aad5147</ref><ref name="Rev431">doi:10.1016/j.molcel.2015.10.008</ref>]] | [[Image:F2r.jpg|left|450px|thumb|Figure. 2. CRISPR diversity and evolution. (A) Modular organization of the CRISPR-Cas systems. LS, large subunit; SS, small subunit. A putative small subunit that might be fused to the large subunit in several type I subtypes is indicated by an asterisk. Cas3 is shown as fusion of two distinct genes encoding the helicase Cas3′ and the nuclease HD Cas3′′; in some type I systems, these domains are encoded by separate genes. Functionally dispensable components are indicated by dashed outlines. Cas6 is shown with a thin solid outline for type I because it is dispensable in some systems, and by a dashed line for type III because most systems lack this gene and use the Cas6 provided in trans by other CRISPR-Cas loci. The two colors for Cas4 and C2c2 and three colors for Cas9 and Cpf1 reflect the contributions of these proteins to different stages of the CRISPR-Cas response (see text). The question marks indicate currently unknown components. From <ref name="Rev4">doi:10.1126/science.aad5147</ref><ref name="Rev430">doi:10.1038/nrmicro3569</ref> (B) Evolutionary scenario for the CRISPR-Cas systems. TR, terminal repeats; TS, terminal sequences; HD, HD-family endonuclease; HNH, HNH-family endonuclease; RuvC, RuvC-family endonuclease; HEPN, putative endoribonuclease of HEPN superfamily. Genes and portions of genes shown in gray denote sequences that are thought to have been encoded in the respective mobile elements but were eliminated in the course of evolution of CRISPR-Cas systems. From <ref name="Rev4">doi:10.1126/science.aad5147</ref><ref name="Rev431">doi:10.1016/j.molcel.2015.10.008</ref>]] | ||
{{Clear}} | {{Clear}} | ||
Revision as of 11:31, 27 November 2016
| |||||||||||
References
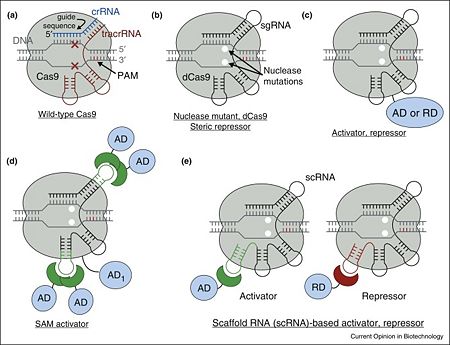
- ↑ 1.0 1.1 1.2 1.3 1.4 Didovyk A, Borek B, Tsimring L, Hasty J. Transcriptional regulation with CRISPR-Cas9: principles, advances, and applications. Curr Opin Biotechnol. 2016 Aug;40:177-84. doi: 10.1016/j.copbio.2016.06.003. Epub, 2016 Jun 23. PMID:27344519 doi:http://dx.doi.org/10.1016/j.copbio.2016.06.003
- ↑ Brophy JA, Voigt CA. Principles of genetic circuit design. Nat Methods. 2014 May;11(5):508-20. doi: 10.1038/nmeth.2926. PMID:24781324 doi:http://dx.doi.org/10.1038/nmeth.2926
- ↑ Straubeta A, Lahaye T. Zinc fingers, TAL effectors, or Cas9-based DNA binding proteins: what's best for targeting desired genome loci? Mol Plant. 2013 Sep;6(5):1384-7. doi: 10.1093/mp/sst075. Epub 2013 May 29. PMID:23718948 doi:http://dx.doi.org/10.1093/mp/sst075
- ↑ Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014 Apr;32(4):347-55. doi: 10.1038/nbt.2842. Epub 2014 Mar 2. PMID:24584096 doi:http://dx.doi.org/10.1038/nbt.2842
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Hochstrasser ML, Doudna JA. Cutting it close: CRISPR-associated endoribonuclease structure and function. Trends Biochem Sci. 2015 Jan;40(1):58-66. doi: 10.1016/j.tibs.2014.10.007. Epub, 2014 Nov 18. PMID:25468820 doi:http://dx.doi.org/10.1016/j.tibs.2014.10.007
- ↑ 6.0 6.1 6.2 Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007 Mar 23;315(5819):1709-12. PMID:17379808 doi:http://dx.doi.org/10.1126/science.1138140
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Mohanraju P, Makarova KS, Zetsche B, Zhang F, Koonin EV, van der Oost J. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science. 2016 Aug 5;353(6299):aad5147. doi: 10.1126/science.aad5147. PMID:27493190 doi:http://dx.doi.org/10.1126/science.aad5147
- ↑ 8.0 8.1 Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadan AH, Moineau S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010 Nov 4;468(7320):67-71. doi: 10.1038/nature09523. PMID:21048762 doi:http://dx.doi.org/10.1038/nature09523
- ↑ Heler R, Marraffini LA, Bikard D. Adapting to new threats: the generation of memory by CRISPR-Cas immune systems. Mol Microbiol. 2014 Jul;93(1):1-9. doi: 10.1111/mmi.12640. Epub 2014 Jun 4. PMID:24806524 doi:http://dx.doi.org/10.1111/mmi.12640
- ↑ Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV. A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct. 2006 Mar 16;1:7. PMID:16545108 doi:http://dx.doi.org/10.1186/1745-6150-1-7
- ↑ 11.0 11.1 Kunin V, Sorek R, Hugenholtz P. Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol. 2007;8(4):R61. PMID:17442114 doi:http://dx.doi.org/10.1186/gb-2007-8-4-r61
- ↑ 12.0 12.1 12.2 Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008 Aug 15;321(5891):960-4. doi: 10.1126/science.1159689. PMID:18703739 doi:http://dx.doi.org/10.1126/science.1159689
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Jiang F, Zhou K, Ma L, Gressel S, Doudna JA. STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition. Science. 2015 Jun 26;348(6242):1477-81. doi: 10.1126/science.aab1452. PMID:26113724 doi:http://dx.doi.org/10.1126/science.aab1452
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012 Aug 17;337(6096):816-21. doi: 10.1126/science.1225829. Epub 2012, Jun 28. PMID:22745249 doi:http://dx.doi.org/10.1126/science.1225829
- ↑ 15.0 15.1 15.2 15.3 Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):E2579-86. Epub 2012 Sep 4. PMID:22949671 doi:http://dx.doi.org/10.1073/pnas.1208507109
- ↑ 16.0 16.1 16.2 16.3 16.4 Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013 Feb 28;152(5):1173-83. doi: 10.1016/j.cell.2013.02.022. PMID:23452860 doi:http://dx.doi.org/10.1016/j.cell.2013.02.022
- ↑ 17.0 17.1 17.2 17.3 Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013 Aug;41(15):7429-37. doi: 10.1093/nar/gkt520. Epub 2013, Jun 12. PMID:23761437 doi:http://dx.doi.org/10.1093/nar/gkt520
- ↑ 18.0 18.1 Kuscu C, Arslan S, Singh R, Thorpe J, Adli M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat Biotechnol. 2014 Jul;32(7):677-83. doi: 10.1038/nbt.2916. Epub 2014 May 18. PMID:24837660 doi:http://dx.doi.org/10.1038/nbt.2916
- ↑ Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJ, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015 Nov;13(11):722-36. doi: 10.1038/nrmicro3569. Epub 2015, Sep 28. PMID:26411297 doi:http://dx.doi.org/10.1038/nrmicro3569
- ↑ Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, Minakhin L, Joung J, Konermann S, Severinov K, Zhang F, Koonin EV. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol Cell. 2015 Nov 5;60(3):385-97. doi: 10.1016/j.molcel.2015.10.008. Epub 2015, Oct 22. PMID:26593719 doi:http://dx.doi.org/10.1016/j.molcel.2015.10.008
- ↑ Wang R, Zheng H, Preamplume G, Shao Y, Li H. The impact of CRISPR repeat sequence on structures of a Cas6 protein-RNA complex. Protein Sci. 2012 Mar;21(3):405-17. doi: 10.1002/pro.2028. Epub 2012 Feb 9. PMID:22238224 doi:http://dx.doi.org/10.1002/pro.2028
- ↑ Shao Y, Li H. Recognition and Cleavage of a Nonstructured CRISPR RNA by Its Processing Endoribonuclease Cas6. Structure. 2013 Feb 27. pii: S0969-2126(13)00017-8. doi:, 10.1016/j.str.2013.01.010. PMID:23454186 doi:http://dx.doi.org/10.1016/j.str.2013.01.010
- ↑ Reeks J, Sokolowski RD, Graham S, Liu H, Naismith JH, White MF. Structure of a dimeric crenarchaeal Cas6 enzyme with an atypical active site for CRISPR RNA processing. Biochem J. 2013 Mar 25. PMID:23527601 doi:10.1042/BJ20130269
- ↑ Niewoehner O, Jinek M, Doudna JA. Evolution of CRISPR RNA recognition and processing by Cas6 endonucleases. Nucleic Acids Res. 2013 Oct 22. PMID:24150936 doi:http://dx.doi.org/10.1093/nar/gkt922
- ↑ Mulepati S, Heroux A, Bailey S. Crystal structure of a CRISPR RNA-guided surveillance complex bound to a ssDNA target. Science. 2014 Aug 14. pii: 1256996. PMID:25123481 doi:http://dx.doi.org/10.1126/science.1256996
- ↑ Hayes RP, Xiao Y, Ding F, van Erp PB, Rajashankar K, Bailey S, Wiedenheft B, Ke A. Structural basis for promiscuous PAM recognition in type I-E Cascade from E. coli. Nature. 2016 Feb 25;530(7591):499-503. doi: 10.1038/nature16995. Epub 2016 Feb, 10. PMID:26863189 doi:http://dx.doi.org/10.1038/nature16995
- ↑ Marraffini LA. CRISPR-Cas immunity in prokaryotes. Nature. 2015 Oct 1;526(7571):55-61. doi: 10.1038/nature15386. PMID:26432244 doi:http://dx.doi.org/10.1038/nature15386
- ↑ Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA. Structures of Cas9 Endonucleases Reveal RNA-Mediated Conformational Activation. Science. 2014 Feb 6. PMID:24505130 doi:http://dx.doi.org/10.1126/science.1247997
- ↑ Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014 Feb 27;156(5):935-49. doi: 10.1016/j.cell.2014.02.001. Epub 2014 Feb, 13. PMID:24529477 doi:http://dx.doi.org/10.1016/j.cell.2014.02.001
- ↑ Jiang F, Taylor DW, Chen JS, Kornfeld JE, Zhou K, Thompson AJ, Nogales E, Doudna JA. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science. 2016 Jan 14. pii: aad8282. PMID:26841432 doi:http://dx.doi.org/10.1126/science.aad8282
- ↑ 31.0 31.1 Nielsen AA, Voigt CA. Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks. Mol Syst Biol. 2014 Nov 24;10:763. doi: 10.15252/msb.20145735. PMID:25422271
- ↑ 32.0 32.1 Didovyk A, Borek B, Hasty J, Tsimring L. Orthogonal Modular Gene Repression in Escherichia coli Using Engineered CRISPR/Cas9. ACS Synth Biol. 2016 Jan 15;5(1):81-8. doi: 10.1021/acssynbio.5b00147. Epub 2015 , Sep 30. PMID:26390083 doi:http://dx.doi.org/10.1021/acssynbio.5b00147
- ↑ 33.0 33.1 Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013 Jul 18;154(2):442-51. doi: 10.1016/j.cell.2013.06.044. Epub 2013 Jul, 11. PMID:23849981 doi:http://dx.doi.org/10.1016/j.cell.2013.06.044
- ↑ 34.0 34.1 Farzadfard F, Perli SD, Lu TK. Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synth Biol. 2013 Oct 18;2(10):604-13. doi: 10.1021/sb400081r. Epub 2013 Sep, 11. PMID:23977949 doi:http://dx.doi.org/10.1021/sb400081r
- ↑ 35.0 35.1 Kiani S, Beal J, Ebrahimkhani MR, Huh J, Hall RN, Xie Z, Li Y, Weiss R. CRISPR transcriptional repression devices and layered circuits in mammalian cells. Nat Methods. 2014 Jul;11(7):723-6. doi: 10.1038/nmeth.2969. Epub 2014 May 5. PMID:24797424 doi:http://dx.doi.org/10.1038/nmeth.2969
![Fig. 1 Overview of the CRISPR-Cas systems. (A) Architecture of class 1 (multiprotein effector complexes) and class 2 (single-protein effector complexes) CRISPR-Cas systems. (B) CRISPR-Cas adaptive immunity is mediated by CRISPR RNAs (crRNAs) and Cas proteins, which form multicomponent CRISPR ribonucleoprotein (crRNP) complexes. The first stage is adaptation, which occurs upon entry of an invading mobile genetic element (in this case, a viral genome). Cas1 (blue) and Cas2 (yellow) proteins select and process the invading DNA, and thereafter, a protospacer (orange) is integrated as a new spacer at the leader end of the CRISPR array [repeat sequences (gray) that separate similar-sized, invader-derived spacers (multiple colors)]. During the second stage, expression, the CRISPR locus is transcribed and the pre-crRNA is processed into mature crRNA guides by Cas (e.g., Cas6) or non-Cas proteins (e.g., RNase III). During the final interference stage, the Cas-crRNA complex scans invading DNA for a complementary nucleic acid target, after which the target is degraded by a Cas nuclease. From](/wiki/images/thumb/f/f2/F1r.jpg/450px-F1r.jpg)