We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 54321
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
== Mechanism == | == Mechanism == | ||
Lisinopril is a drug used mainly to treat hypertension but also to reduce the risk of long-term damage and death in patients suffering from heart failure. Lisinopril controls blood pressure by inhibiting the angiotensin converting enzyme (ACE) and ceasing the degradation of Bradykinin (Bk), a vasodilator, signaled by the presence of ATII. By inhibiting ACE, it inevitably prevents the body from synthesizing angiotensin II. Without the surplus of angiotensin II being made, the blood pressure in the body lowers due to the increase in the ratio of vasodilating angiotensin I to vasoconstricting angiotensin II and preventing the inhibition of Bk.<ref>Helen, Allen(2016). Lisinopril: Lisinopril ACE inhibitor. Patient. Retrieved from: http://patient.info/medicine/lisinopril-an-ace-inhibitor-zestril</ref> | Lisinopril is a drug used mainly to treat hypertension but also to reduce the risk of long-term damage and death in patients suffering from heart failure. Lisinopril controls blood pressure by inhibiting the angiotensin converting enzyme (ACE) and ceasing the degradation of Bradykinin (Bk), a vasodilator, signaled by the presence of ATII. By inhibiting ACE, it inevitably prevents the body from synthesizing angiotensin II. Without the surplus of angiotensin II being made, the blood pressure in the body lowers due to the increase in the ratio of vasodilating angiotensin I to vasoconstricting angiotensin II and preventing the inhibition of Bk.<ref>Helen, Allen(2016). Lisinopril: Lisinopril ACE inhibitor. Patient. Retrieved from: http://patient.info/medicine/lisinopril-an-ace-inhibitor-zestril</ref> | ||
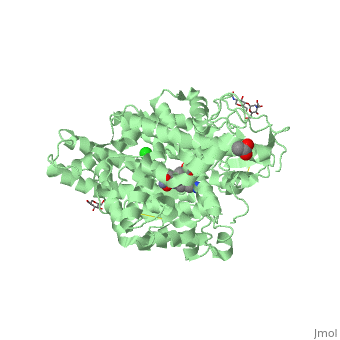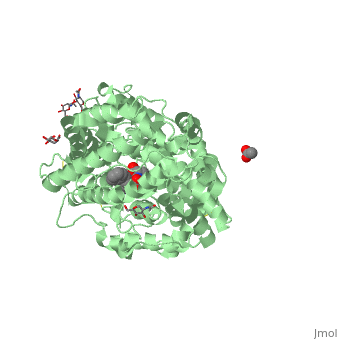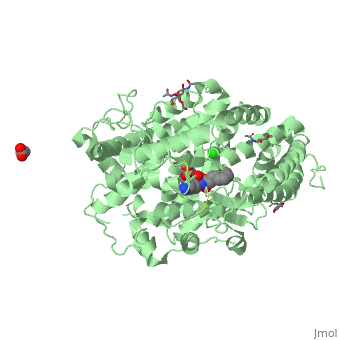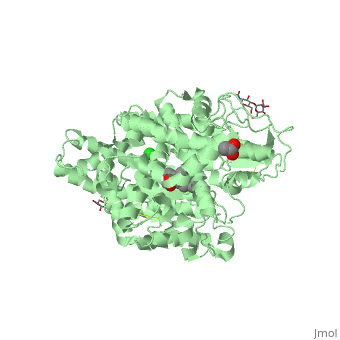
| - | There are two types of membrane associated ACE proteins within the body. Coded by the same gene, the somatic and testis ACEs are closely similar though display some differences. The testes ACE protein consists of two domains which include their own individual HEXXH <scene name='74/745973/Zinc_binding_motif/ | + | There are two types of membrane associated ACE proteins within the body. Coded by the same gene, the somatic and testis ACEs are closely similar though display some differences. The testes ACE protein consists of two domains which include their own individual HEXXH <scene name='74/745973/Zinc_binding_motif/4'>zinc binding motif</scene> which forms many ligands that catalyze the hydrolysis of many mechanisms. In respect to Lisinopril, the zinc ions are involved with the hydrolysis of angiotensin I where the His-Leu dipeptide residues are cleaved at the C domain which restricts access to the active site. The binding position in the C domains controls the conversion of angiotensin. Therefor when Lisinopril binds, ACE becomes distorted and curved. This curved and helical structure is known as tACE. This helical structure is formed via the chloride ions near the active site which begin substrate hydrolysis in the C domain. Lisinopril is known to bind to the individual HEXXH zinc binding motif in the lysine side chain in addition the extended phenyl group near the active site. <ref>Natesh, R., Schwager, S.L.U., Sturrock, E.D., Acharya, K. R. (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex.Nature 421, 551-554 doi:10.1038/nature01370</ref>. |
The somatic angiotensin converting enzyme, or sACE, contains two highly functional active sites at the C terminus and N terminus. The C terminus of sACE and tACE are highly analogous, though each sACE terminus bears the zinc binding domain HEMGH, different from tACE’s HEXXH. Each sACE domain acts to convert ATI to ATII and to inactivate Bk. However, relative to the N terminus, the C terminus of the somatic enzyme is known to be less chemically stable and more reactive to environmental Cl- ions than its counter. Lisinopril acts with specificity to block the N and C terminus active sites.It competitively binds to sACE via hydrogen bonds, stacking of aromatic rings, and ionic interactions. Both the hydrophilic portions and hydrophobic aromatic rings of tyrosine and phenylalanine residues at the C-terminus of sACe interact with Lisinopril at the S2 binding site, coupling to create more favorable reactions. The aromatic rings present throughout the amino acid strand create a favorable nonpolar environment while those facets such as the hydrophilic ends of Tyr527,532,1123,1138 and Phe466,521,1062,1117, specifically, allow for the molecule to interact with different types of substrate. The N-terminus of sACE, however, forms weaker and less favorable interactions between the sACE and ACE inhibitor relative to the C-terminus. A salt bridge formation between Glu residues of sACE allow Lisinopril access to its <scene name='74/745973/Lisinopril_binding/1'>active site</scene>, compensating for the relatively weak interactions of the N-terminus end <ref>Fernandez, J., Hayashi, M., Camargo, A., Neshich, G. (2003) Biochemical and Biophysical Research Communications. Volume 308. Pages 219-226.</ref> | The somatic angiotensin converting enzyme, or sACE, contains two highly functional active sites at the C terminus and N terminus. The C terminus of sACE and tACE are highly analogous, though each sACE terminus bears the zinc binding domain HEMGH, different from tACE’s HEXXH. Each sACE domain acts to convert ATI to ATII and to inactivate Bk. However, relative to the N terminus, the C terminus of the somatic enzyme is known to be less chemically stable and more reactive to environmental Cl- ions than its counter. Lisinopril acts with specificity to block the N and C terminus active sites.It competitively binds to sACE via hydrogen bonds, stacking of aromatic rings, and ionic interactions. Both the hydrophilic portions and hydrophobic aromatic rings of tyrosine and phenylalanine residues at the C-terminus of sACe interact with Lisinopril at the S2 binding site, coupling to create more favorable reactions. The aromatic rings present throughout the amino acid strand create a favorable nonpolar environment while those facets such as the hydrophilic ends of Tyr527,532,1123,1138 and Phe466,521,1062,1117, specifically, allow for the molecule to interact with different types of substrate. The N-terminus of sACE, however, forms weaker and less favorable interactions between the sACE and ACE inhibitor relative to the C-terminus. A salt bridge formation between Glu residues of sACE allow Lisinopril access to its <scene name='74/745973/Lisinopril_binding/1'>active site</scene>, compensating for the relatively weak interactions of the N-terminus end <ref>Fernandez, J., Hayashi, M., Camargo, A., Neshich, G. (2003) Biochemical and Biophysical Research Communications. Volume 308. Pages 219-226.</ref> | ||
Revision as of 21:25, 5 December 2016
| |||||||||||
References
- ↑ National Center for Biotechnology Information. PubChem Compound Database; CID=5362119, https://pubchem.ncbi.nlm.nih.gov/compound/5362119
- ↑ Helen, Allen (2016). Lisinopril: Lisinopril ACE inhibitor. Patient. Retrieved from: http://patient.info/medicine/lisinopril-an-ace-inhibitor-zestril
- ↑ National Center for Biotechnology Information. PubChem Compound Database; CID=5362119, https://pubchem.ncbi.nlm.nih.gov/compound/5362119 (accessed Nov. 12, 2016).
- ↑ Bouabdallah, S., Dhia, T. B., & Driss, R. (2014, February 25). Study of a Conformational Equilibrium of Lisinopril by HPLC, NMR, and DFT. Retrieved November 12, 2016, from https://www.hindawi.com/journals/ijac/2014/494719/
- ↑ Helen, Allen(2016). Lisinopril: Lisinopril ACE inhibitor. Patient. Retrieved from: http://patient.info/medicine/lisinopril-an-ace-inhibitor-zestril
- ↑ Natesh, R., Schwager, S.L.U., Sturrock, E.D., Acharya, K. R. (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex.Nature 421, 551-554 doi:10.1038/nature01370
- ↑ Fernandez, J., Hayashi, M., Camargo, A., Neshich, G. (2003) Biochemical and Biophysical Research Communications. Volume 308. Pages 219-226.