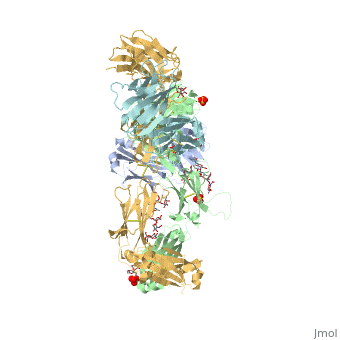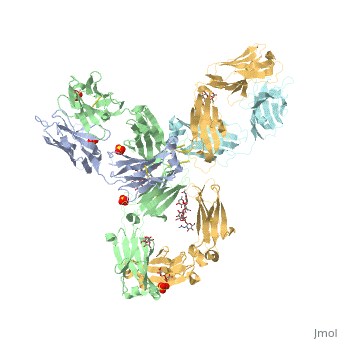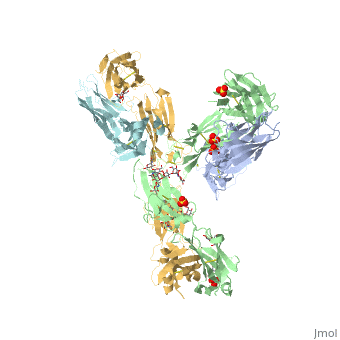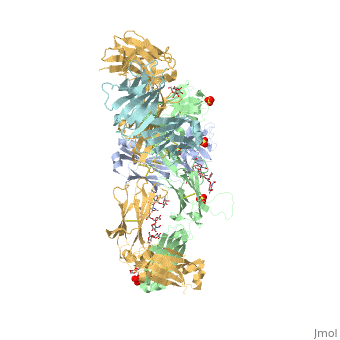We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Keytruda
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Mechanism == | == Mechanism == | ||
| - | + | ||
===Pembrolizumab/PD-1 Interaction=== | ===Pembrolizumab/PD-1 Interaction=== | ||
| - | In order for Pembrolizumab to block PD-1, Pembrolizumab forms a large, flat paratope (antigen-binding site) that can sustain PD-1’s large epitope (where antibody attaches on antigen). The induced interaction between Pembrolizumab and PD-1 gives rise to a surface conformational change on PD-1. The new structure of PD-1 becomes a very shallow, “crescent”-like shape, in contrast to the flat conformation when bound to PD-L1 <ref name="horita">DOI:10.1038/srep35297</ref>. | + | In order for Pembrolizumab to block PD-1, Pembrolizumab forms a large, flat paratope (antigen-binding site) that can sustain PD-1’s large epitope (where antibody attaches on antigen). The induced [http://www.nature.com/articles/srep35297/figures/2 interaction between Pembrolizumab and PD-1] gives rise to a surface conformational change on PD-1. The new structure of PD-1 becomes a very shallow, “crescent”-like shape, in contrast to the flat conformation when bound to PD-L1 <ref name="horita">DOI:10.1038/srep35297</ref>. |
===PemFv/PD-1 Interaction=== | ===PemFv/PD-1 Interaction=== | ||
The Fv fragment of Pembrolizumab can form a complex with the extracellular domain (ECD) of PD-1. Both PemFv and PD-1<sub>ECD</sub> contain interchain disulfide bonds. PemFv interacts predominantly in the major groove of PD-1, which is formed on one surface by the CC’FG antiparallel β−sheet and the BC, C’D, and FG loops. There are 15 direct hydrogen bonds between the residues, 15 water-mediated hydrogen bonds, 2 salt bridges, and many hydrophobic interactions. There are a total of 26 PD-1<sub>ECD</sub> residues involved in the interaction with PemFv, with residues in loop C’D (Pro84 to Gly90) and strand C’ (Gln75 to Lys 78) playing a major role. These key components of PD-1 mainly form interactions through salt bridges and hydrogen bonds with complementary determining regions, the variable domains, of Pembrolizumab. <scene name='74/745945/Chain_b_amino_acids/1'>Thr30, Tyr33, Ser54, Tyr101, Arg102</scene> on Chain B of Pembrolizumab form bonds with Asp77, Gln75, Lys78, Thr76, Tyr68, and Asn66 of PD-1. It is believed that the sugar chains of PD-1 have no physical contact with Pembrolizumab due to the N-linked glycosylated residues (Asn49, Asn58, Asn74, and Asn116) being located away from the interface <ref name="horita" />. | The Fv fragment of Pembrolizumab can form a complex with the extracellular domain (ECD) of PD-1. Both PemFv and PD-1<sub>ECD</sub> contain interchain disulfide bonds. PemFv interacts predominantly in the major groove of PD-1, which is formed on one surface by the CC’FG antiparallel β−sheet and the BC, C’D, and FG loops. There are 15 direct hydrogen bonds between the residues, 15 water-mediated hydrogen bonds, 2 salt bridges, and many hydrophobic interactions. There are a total of 26 PD-1<sub>ECD</sub> residues involved in the interaction with PemFv, with residues in loop C’D (Pro84 to Gly90) and strand C’ (Gln75 to Lys 78) playing a major role. These key components of PD-1 mainly form interactions through salt bridges and hydrogen bonds with complementary determining regions, the variable domains, of Pembrolizumab. <scene name='74/745945/Chain_b_amino_acids/1'>Thr30, Tyr33, Ser54, Tyr101, Arg102</scene> on Chain B of Pembrolizumab form bonds with Asp77, Gln75, Lys78, Thr76, Tyr68, and Asn66 of PD-1. It is believed that the sugar chains of PD-1 have no physical contact with Pembrolizumab due to the N-linked glycosylated residues (Asn49, Asn58, Asn74, and Asn116) being located away from the interface <ref name="horita" />. | ||
Revision as of 02:53, 6 December 2016
Pembrolizumab antibody against programmed cell death-1 receptor
| |||||||||||
References
- ↑ 1.0 1.1 Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol. 2016 Oct;12(10):1247-53. doi:, 10.1080/17425255.2016.1216976. Epub 2016 Aug 16. PMID:27485741 doi:http://dx.doi.org/10.1080/17425255.2016.1216976
- ↑ 2.0 2.1 2.2 2.3 Horita S, Nomura Y, Sato Y, Shimamura T, Iwata S, Nomura N. High-resolution crystal structure of the therapeutic antibody pembrolizumab bound to the human PD-1. Sci Rep. 2016 Oct 13;6:35297. doi: 10.1038/srep35297. PMID:27734966 doi:http://dx.doi.org/10.1038/srep35297
- ↑ Rajakulendran T, Adam DN. Spotlight on pembrolizumab in the treatment of advanced melanoma. Drug Des Devel Ther. 2015 Jun 4;9:2883-6. doi: 10.2147/DDDT.S78036. eCollection, 2015. PMID:26082618 doi:http://dx.doi.org/10.2147/DDDT.S78036
- ↑ Deeks ED. Pembrolizumab: A Review in Advanced Melanoma. Drugs. 2016 Mar;76(3):375-86. doi: 10.1007/s40265-016-0543-x. PMID:26846323 doi:http://dx.doi.org/10.1007/s40265-016-0543-x