Sandbox 45673
From Proteopedia
| Line 15: | Line 15: | ||
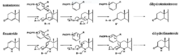
[[Image: Biochem group project.PNG|thumb|left|'''Fig. 1'''. Proposed mechanism of inhibition by Bull et. al. Image shows how the inhibition of 5alpha-reductase is achieved as Finasteride is used as a substrate to create an enolate intermediate, similar to the one made during the reduction of testosterone. However, the complex created does not allow for the proton transfer needed to complete the reduction of NADP-Dihydrofinasteride to Finasteride, because of the change in the carbanion position<ref name="five">Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t</ref>]] | [[Image: Biochem group project.PNG|thumb|left|'''Fig. 1'''. Proposed mechanism of inhibition by Bull et. al. Image shows how the inhibition of 5alpha-reductase is achieved as Finasteride is used as a substrate to create an enolate intermediate, similar to the one made during the reduction of testosterone. However, the complex created does not allow for the proton transfer needed to complete the reduction of NADP-Dihydrofinasteride to Finasteride, because of the change in the carbanion position<ref name="five">Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t</ref>]] | ||
| - | ==Medical== | + | ==Medical Uses== |
Finasteride is used to shrink an enlarged prostate, also known as benign prostatic hyperplasia (BPH), in adult men (Allen, 2015). Enlarged prostate is located near the bladder which causes difficulty with passing urine. Common symptoms include, long period of time before urine flow, feeling the bladder is not empty, and dribbling urine (Allen, 2015). This medication works by blocking the enzyme 5α-reductase, which prevents conversion of testosterone to the natural body hormone, dihydrosestosterone (DHT) that causes growth of the prostate. As a result, reducing the amount of dihydrotestosterone causes the prostate to shrink. Thus, helps the urine pass easily. *I plan to add hyperlinks* | Finasteride is used to shrink an enlarged prostate, also known as benign prostatic hyperplasia (BPH), in adult men (Allen, 2015). Enlarged prostate is located near the bladder which causes difficulty with passing urine. Common symptoms include, long period of time before urine flow, feeling the bladder is not empty, and dribbling urine (Allen, 2015). This medication works by blocking the enzyme 5α-reductase, which prevents conversion of testosterone to the natural body hormone, dihydrosestosterone (DHT) that causes growth of the prostate. As a result, reducing the amount of dihydrotestosterone causes the prostate to shrink. Thus, helps the urine pass easily. *I plan to add hyperlinks* | ||
Revision as of 03:09, 6 December 2016
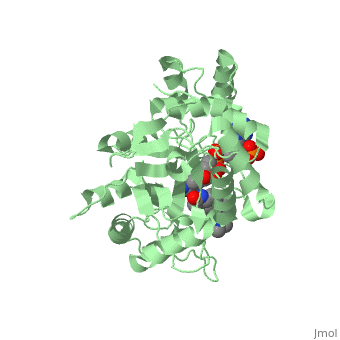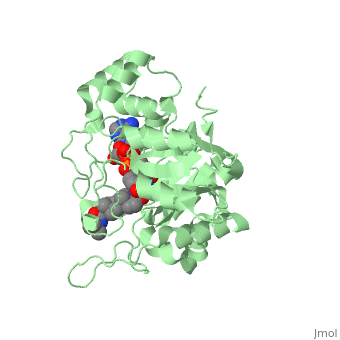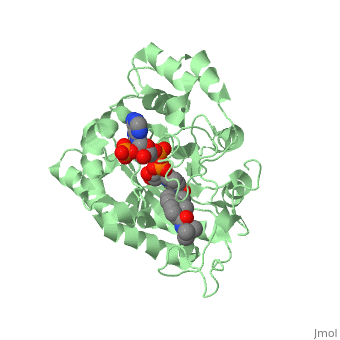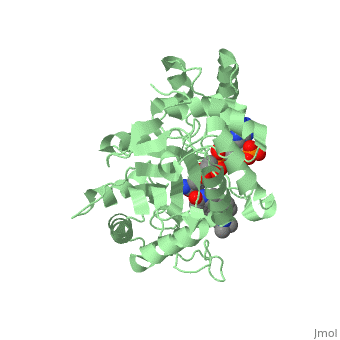
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide
| |||||||||||
References
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3
- ↑ Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9
- ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
Allen, Helen. (2015, March). "Finasteride for prostate gland enlargement. Information. Patient.
Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
Leyden, J., Dunlap, F., & Miller, B., et el. (1999, June). Finasteride in the treatment of men with frontal male pattern hair loss.
Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride.
Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm