We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 45673
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
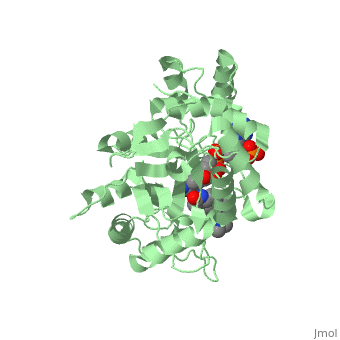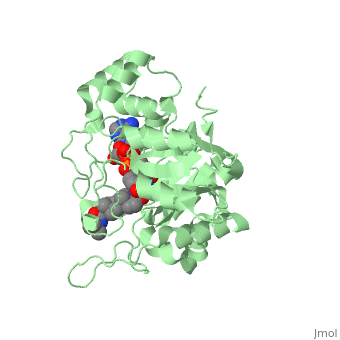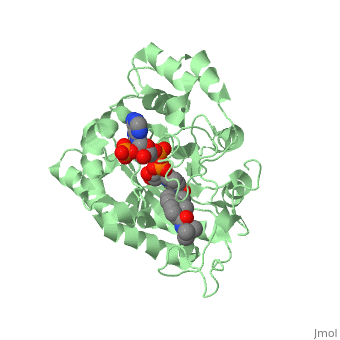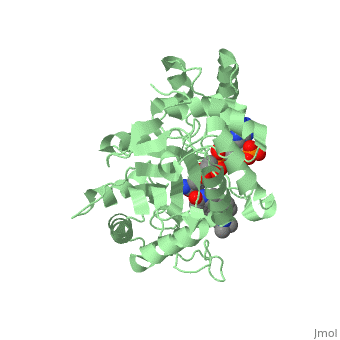
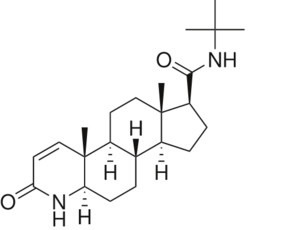
<StructureSection load='3G1R' size='350' side='right' scene='' caption='N-(1,1-dimethylethyl)-3-oxo- | <StructureSection load='3G1R' size='350' side='right' scene='' caption='N-(1,1-dimethylethyl)-3-oxo- | ||
| - | (5α,17β)-4-azaandrost-1-ene-17-carboxamide bound to | + | (5α,17β)-4-azaandrost-1-ene-17-carboxamide bound to 5β-reductase (PDB code [[3g1r]])'> |
==Function== | ==Function== | ||
Revision as of 05:31, 6 December 2016
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide (Finasteride)
| |||||||||||
References
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ 4.0 4.1 Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3
- ↑ Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9
- ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
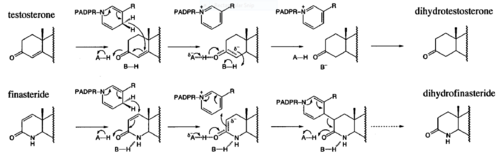
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
- ↑ name="twenty" Allen, Helen. (2015, March). "Finasteride for prostate gland enlargement”. Information. Patient. http://patient.info/medicine/finasteride-for-prostate-gland-enlargement-proscar
- ↑ name="twenty" Allen, Helen. (2015, March). "Finasteride for prostate gland enlargement”. Information. Patient. http://patient.info/medicine/finasteride-for-prostate-gland-enlargement-proscar
- ↑ Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride.
- ↑ Leyden, James et al.(June 1999)."Finasteride in the treatment of men with frontal male pattern hair loss." Journal of the American Academy of Dermatology. Volume 40 , Issue 6 , 930 - 937