We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 45673
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
'''Benign Prostatic Hyperplasia (BPH)''' | '''Benign Prostatic Hyperplasia (BPH)''' | ||
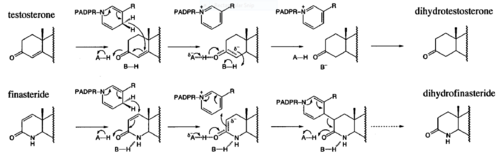
| - | Aromatase and 5α-reductase is responsible for converting androgen hormones into estrogen and dihydrotestosterone (DHT). This chemical process of androgen hormones leads to a decrease in testosterone, but raises levels of DHT and estrogen. Estrogen is a key role in cells proliferating in the prostate and DHT is an anabolic hormone much more potent (dissociated from the androgen receptor slowly) than testosterone that when combined, causes a synergy to induce BPH. The enzyme 5α-reductase is responsible for the synthesis of DHT in the prostate from circulating testosterone. 5α-reductase is located in the stromal cells, which is the main site for the synthesis of DHT, but it can also diffuse into epithelial cells close-by. In both stromal and epithelial cells, DHT binds to nuclear androgen receptors and signals for transcription for cell growth. Finasteride is used to inhibit the 5α-reductase enzyme, which blocks the conversion of testosterone and inhibits the production of DHT, reducing prostate volume and BPH symptoms (urinating complication). Using finasteride could increase the risk for erectile dysfunction, decrease libido, and ejaulation disorder due to 5α-reductase being inhibited. | + | [[Aromatase]] and 5α-reductase is responsible for converting androgen hormones into estrogen and dihydrotestosterone (DHT). This chemical process of androgen hormones leads to a decrease in testosterone, but raises levels of DHT and estrogen. Estrogen is a key role in cells proliferating in the prostate and DHT is an anabolic hormone much more potent (dissociated from the androgen receptor slowly) than testosterone that when combined, causes a synergy to induce BPH. The enzyme 5α-reductase is responsible for the synthesis of DHT in the prostate from circulating testosterone. 5α-reductase is located in the stromal cells, which is the main site for the synthesis of DHT, but it can also diffuse into epithelial cells close-by. In both stromal and epithelial cells, DHT binds to nuclear androgen receptors and signals for transcription for cell growth. Finasteride is used to inhibit the 5α-reductase enzyme, which blocks the conversion of testosterone and inhibits the production of DHT, reducing prostate volume and BPH symptoms (urinating complication). Using finasteride could increase the risk for erectile dysfunction, decrease libido, and ejaulation disorder due to 5α-reductase being inhibited. |
Revision as of 06:39, 6 December 2016
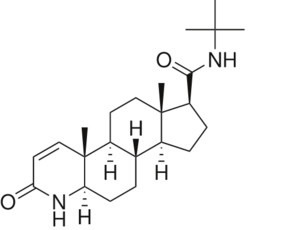
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide (Finasteride)
| |||||||||||
References
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ 4.0 4.1 Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3
- ↑ Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9
- ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
- ↑ Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride.
- ↑ Leyden, James et al.(June 1999)."Finasteride in the treatment of men with frontal male pattern hair loss." Journal of the American Academy of Dermatology. Volume 40 , Issue 6 , 930 - 937