We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 45673
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
==Mechanism== | ==Mechanism== | ||
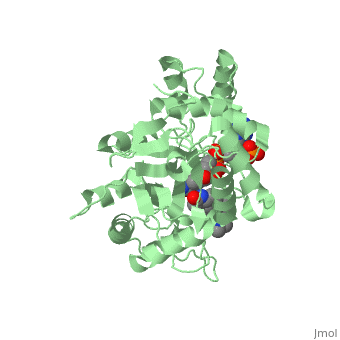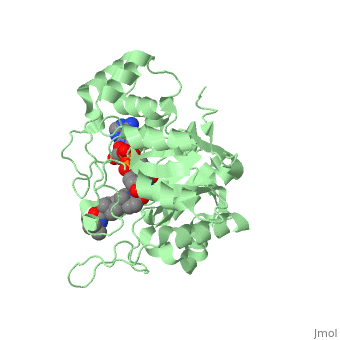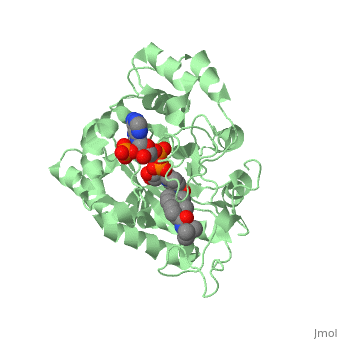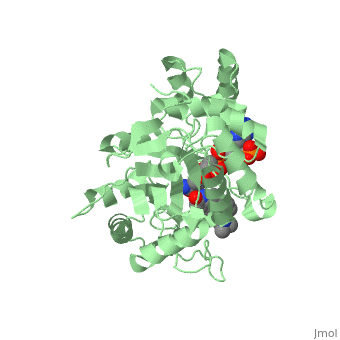
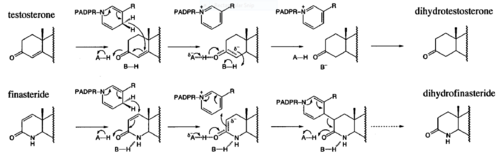
| - | Finasteride is a 5α-reductase inhibitor. There are three isoforms of 5α-reductase, types I, II, and III. While the drug has a higher affinity for the type II enzyme, it also inhibits the function of the type I and no affinity for type III.<ref name="four"> Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm </ref>Typically 5α-redcutase turns testosterone into Dihydrotestosterone(DHT), but the enzyme will accept Finasteride as an alternate substrate; turning it into dihydrofinasteride through an enzyme bound, NADP-dihydrofinasteride adduct. Finasteride is similar in structure to testosterone and | + | Finasteride is a 5α-reductase inhibitor. There are three isoforms of 5α-reductase, types I, II, and III. While the drug has a higher affinity for the type II enzyme, it also inhibits the function of the type I and no affinity for type III.<ref name="four"> Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm </ref>Typically 5α-redcutase turns testosterone into Dihydrotestosterone(DHT), but the enzyme will accept Finasteride as an alternate substrate; turning it into dihydrofinasteride through an enzyme bound, NADP-dihydrofinasteride adduct. Finasteride is similar in structure to testosterone and 5α-reductase has almost the same affinity for both molecules. However, Finasteride , having a high affinity for 5α-reductase, covalently binds to the enzyme as a Michael acceptor, through a functionally irreversible reaction. However, the NADP-dihydrofinasteride complex breaks down with a half life of about 1 month at 37˚C., which is why patients must continue taking the drug.<ref name="five"> Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t</ref> |
[[Image: Biochem group project.PNG|thumb|500px|right|'''Fig. 2'''. Proposed mechanism of inhibition by Bull et. al. Image shows how the inhibition of 5alpha-reductase is achieved as Finasteride is used as a substrate to create an enolate intermediate, similar to the one made during the reduction of testosterone. However, the complex created does not allow for the proton transfer needed to complete the reduction of NADP-Dihydrofinasteride to Finasteride, because of the change in the carbanion position<ref name="five">Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t</ref>]] | [[Image: Biochem group project.PNG|thumb|500px|right|'''Fig. 2'''. Proposed mechanism of inhibition by Bull et. al. Image shows how the inhibition of 5alpha-reductase is achieved as Finasteride is used as a substrate to create an enolate intermediate, similar to the one made during the reduction of testosterone. However, the complex created does not allow for the proton transfer needed to complete the reduction of NADP-Dihydrofinasteride to Finasteride, because of the change in the carbanion position<ref name="five">Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t</ref>]] | ||
Revision as of 06:54, 6 December 2016
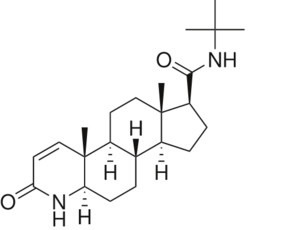
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide (Finasteride)
| |||||||||||
References
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ 4.0 4.1 Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3
- ↑ Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9
- ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
- ↑ Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride.
- ↑ Leyden, James et al.(June 1999)."Finasteride in the treatment of men with frontal male pattern hair loss." Journal of the American Academy of Dermatology. Volume 40 , Issue 6 , 930 - 937