Wiskott-Aldrich syndrome protein
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
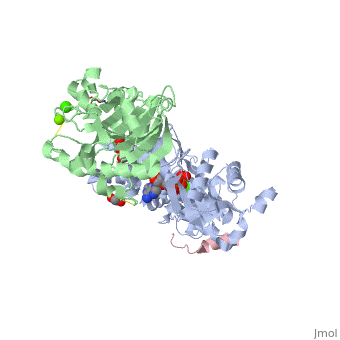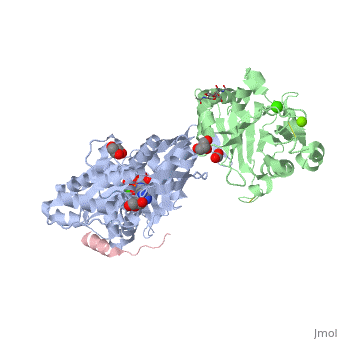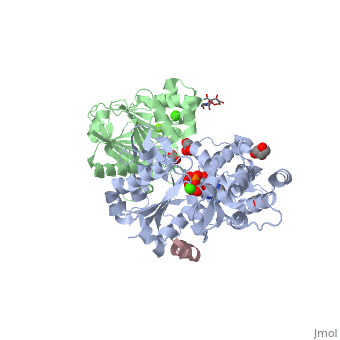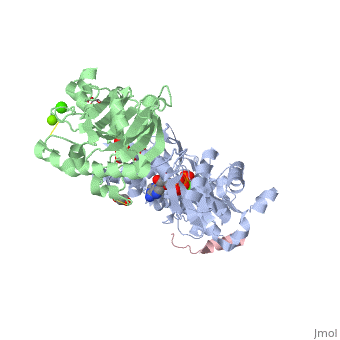
<StructureSection load='2a3z' size='340' side='right' caption='Human WASP WH2 domain (pink) complex with α-actin (grey), DNAse I (green), ATP (stick model), Ca+2 and Mg+2 ions, glycerol and formic acid (PDB code [[2a3z]])' scene=''> | <StructureSection load='2a3z' size='340' side='right' caption='Human WASP WH2 domain (pink) complex with α-actin (grey), DNAse I (green), ATP (stick model), Ca+2 and Mg+2 ions, glycerol and formic acid (PDB code [[2a3z]])' scene=''> | ||
== Function == | == Function == | ||
| - | '''Wiskott-Aldrich syndrome protein''' (WASP) is involved in nucleating of new F-actin. In the autoinhibited form of WASP a region of the N-terminal interacts with a region of its C-terminal. This interaction is disrupted by CDC42 and phosphatidylinositol 4,5-bisphosphate (PIP2) resulting in the active WASP. WASP domains include '''EVH1''' domain in the N-terminal which bind proline-rich sequences in the WASP-interacting proteins; '''WH2''' domain is ca. 18 residues long and interacts with actin; '''CRIB or GTPase-binding''' domain which interacts with CDC42. '''N-WASP''' (Neural WASP) belongs to the WASP family of proteins. Human N-WASP stimulates the actin-nucleating activity<ref>PMID:22411869</ref>. N-WASP induces actin polymerization in the Shigella bacterium. This bacterium causes dysentery. | + | '''Wiskott-Aldrich syndrome protein''' (WASP) is involved in nucleating of new F-actin. In the autoinhibited form of WASP a region of the N-terminal interacts with a region of its C-terminal. This interaction is disrupted by CDC42 and phosphatidylinositol 4,5-bisphosphate (PIP2) resulting in the active WASP. WASP domains include '''EVH1''' domain in the N-terminal which bind proline-rich sequences in the WASP-interacting proteins; '''WH2''' domain is ca. 18 residues long and interacts with actin; '''CRIB or GTPase-binding''' domain which interacts with CDC42. '''N-WASP''' (Neural WASP) belongs to the WASP family of proteins. Human N-WASP stimulates the actin-nucleating activity<ref>PMID:22411869</ref>. |
| + | |||
| + | == Relevance == | ||
| + | N-WASP induces actin polymerization in the Shigella bacterium<ref>PMID:11952639</ref>. This bacterium causes dysentery. | ||
== Disease == | == Disease == | ||
Revision as of 10:36, 14 December 2016
| |||||||||||
3D Structures of Wiskott-Aldrich syndrome protein
Updated on 14-December-2016
References
- ↑ Westerberg LS, Dahlberg C, Baptista M, Moran CJ, Detre C, Keszei M, Eston MA, Alt FW, Terhorst C, Notarangelo LD, Snapper SB. Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B-cell development and function. Blood. 2012 Apr 26;119(17):3966-74. doi: 10.1182/blood-2010-09-308197. Epub 2012 , Mar 12. PMID:22411869 doi:http://dx.doi.org/10.1182/blood-2010-09-308197
- ↑ Suzuki T, Mimuro H, Suetsugu S, Miki H, Takenawa T, Sasakawa C. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell Microbiol. 2002 Apr;4(4):223-33. PMID:11952639
- ↑ Notarangelo LD, Miao CH, Ochs HD. Wiskott-Aldrich syndrome. Curr Opin Hematol. 2008 Jan;15(1):30-6. PMID:18043243 doi:http://dx.doi.org/10.1097/MOH.0b013e3282f30448