We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
CRISPR-Cas Part II
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='4qyz' size='450' side='right' scene='74/742625/Cv/4' caption=''> | <StructureSection load='4qyz' size='450' side='right' scene='74/742625/Cv/4' caption=''> | ||
SEE [[CRISPR-Cas|CRISPR-Cas Part I]] | SEE [[CRISPR-Cas|CRISPR-Cas Part I]] | ||
| - | ==CRISPR adaptation ( | + | |
| + | ==CRISPR adaptation== | ||
| + | |||
| + | The spacers of a CRISPR array represent a chronological archive of previous invader encounters. The captured spacer sequences are integrated into the CRISPR loci after exposure to MGEs, at the leader end of the array that contains the start site of CRISPR transcription. Analysis of invader target sequences (also called protospacers) has revealed a short motif directly adjacent to the target sequence, called the protospacer adjacent motif (PAM). This PAM motif allows self/nonself discrimination by the host in two ways: (i) because its presence in alien targets is required for nonself interference, and (ii) because its absence in the host’s CRISPR array avoids self-targeting. In class 1–type I and class 2–type II systems, the PAM is not only involved in interference, but also plays a role in spacer selection during the adaptation stage, implying the acquisition of functional spacers only. The PAM is a short [2 to 7 nucleotides (nt)], partially redundant sequence that in itself cannot preclude incorporation of spacers from the host DNA because of the low information content of the motif. The short PAM appears to be the result of an evolutionary trade-off between efficient incorporation of spacers from nonself DNA and preventing an autoimmune reaction.<ref name="Rev4">doi:10.1126/science.aad5147</ref> | ||
| + | |||
| + | ''Examples of PAM:'' | ||
| + | *<scene name='74/746096/Cv6/3'>PAM-complementary dual-forked DNA, which is a 23-mer palindromic duplex</scene> from ''Escherichia coli'' ([[5dqz]]). | ||
| + | *<scene name='74/742625/Cv2/10'>PAM in crystal structure of Acidaminococcus sp. Cpf1 in complex with crRNA and target DNA</scene> ([[5b43]]). | ||
| + | *<scene name='74/742625/Cv2/9'>PAM in crRNA-dsDNA hybrid from E. coli</scene> ([[5h9f]]). | ||
| + | *<scene name='74/742625/Cv/42'>PAM in Cas9-sgRNA-target DNA complex from Streptococcus pyogenes</scene> ([[5fw2]]). | ||
| + | *<scene name='74/742625/Cv2/13'>PAM in Cas9-sgRNA-target DNA complex from Francisella tularensis</scene> ([[5b2p]]). | ||
| + | *<scene name='74/742625/Cv3/2'>PAM in Cas9-sgRNA-target DNA complex from Staphylococcus aureus</scene> ([[4axw]]). | ||
| + | *<scene name='74/742625/Cv3/10'>Few nucleotide long conserved motif recognized directly by Cas9 protein (protospacer adjacent motif, PAM)</scene>. | ||
| + | |||
| + | Although host chromosomal fragments can be incorporated as new CRISPR spacers, detection of such events obviously implies that this did not result in a lethal phenotype, either due to a modified PAM and/or to an inactivated CRISPR-Cas effector module <ref name="Rev451">doi:10.1016/ | ||
| + | j.tig.2010.05.008</ref>. Indeed, in the absence of the effector module, elevated frequencies of self-spacer acquisition occur in ''Escherichia coli'' <ref name="Rev452">doi:10.1093/nar/gks216</ref>. Similarly, ''Streptococcus thermophilus'' with a catalytically inactive Cas9 results in a major increase of spacers derived from the host genome <ref name="Rev453">doi:10.1101/gad.257550.114</ref>. In addition, there is a strong preference for the integration of plasmid over chromosomal spacer sequences <ref name="Rev452">doi:10.1093/nar/gks216</ref>, with plasmid sequences incorporated more frequently than host DNA by two to three orders of magnitude <ref name="Rev456">doi:10.1038/nature14302</ref>. Spacer acquisition in ''E. coli'' requires active replication of the protospacercontaining DNA <ref name="Rev456">doi:10.1038/nature14302</ref>. Thus, small, fast-replicating plasmid genomes are a much better source of spacers than the large host DNA, and such findings are consistent with acquisition of spacers from an infecting virus genome in the archaeon ''Sulfolobus islandicus'' requiring its active replication <ref name="Rev457">doi:10.1111/mmi.12503</ref>. In ''E. coli'', the CRISPR-Cas system derives the spacers primarily from products of RecBCD-catalyzed DNA degradation that are formed during the repair of double-stranded breaks associated with stalled replication forks <ref name="Rev458">doi:10.1016/j.cell.2007.11.004</ref>. Other possible sources of substrates for CRISPR adaptation include DNA fragments generated | ||
| + | either by other defense systems, such as restriction-modification systems <ref name="Rev459">doi:10.1038/ncomms3087</ref>, or by the CRISPR-Cas system itself <ref name="Rev449">doi:10.1371/journal.pone.0035888</ref>.<ref name="Rev4">doi:10.1126/science.aad5147</ref> | ||
| + | |||
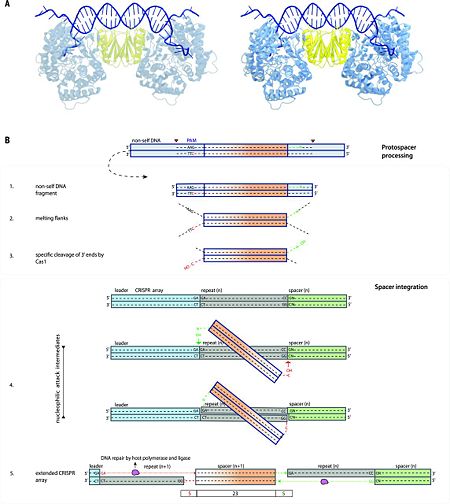
| + | <scene name='74/746096/Cv6/4'>Cas1 and Cas2</scene> play crucial roles in spacer acquisition in all CRISPR-Cas systems <ref name="Rev452">doi:10.1093/nar/gks216</ref>. In addition, these proteins can function in trans, provided that the repeats involved are sufficiently similar in size and structure. Accordingly, ''cas1'' and ''cas2'' genes are missing in many active CRISPR-Cas loci—in particular, of type III as well as types IV and VI <ref name="Rev430">doi:10.1038/nrmicro3569</ref>. Overexpression of Cas1 and Cas2 from the ''E. coli'' type I-E system has been shown to be sufficient for the extension of the CRISPR array <ref name="Rev452">doi:10.1093/nar/gks216</ref>. Mutations in the active site of Cas1 abolish spacer integration in ''E. coli'' <ref name="Rev452">doi:10.1093/nar/gks216</ref>, whereas the nuclease activity of Cas2 is dispensable <ref name="Rev455">doi:10.1038/nsmb.2820</ref>. In ''E. coli'', a <scene name='74/746096/Cv6/5'>central Cas2 dimer and two flanking Cas1 dimers form a complex that binds and processes PAM containing DNA fragments</scene> ([[5dqz]]; Fig. 3A <ref name="Rev455">doi:10.1038/nsmb.2820</ref>, <ref name="Rev460">doi:10.1016/j.cell.2015.10.008</ref>), after which the newly generated spacers can be integrated into a CRISPR array via a recombination mechanism akin to that of retroviral integrases and transposases <ref name="Rev461">doi:10.1038/nature14237</ref> (Fig. 3B). <scene name='74/746096/Cv6/7'>Cas1 cleaves the phosphodiester bond between nucleotides 28 and 29</scene>, resulting in a DNA cleavage product. <scene name='74/746096/Cv6/8'>Glu141, His208, and Asp221 are the catalytic residues of Cas1</scene>. The DNA cleavage site is labeled in red. | ||
| + | |||
| + | [[Image:F4large.jpg|left|450px|thumb|Fig. 3 Spacer acquisition. (A) Crystal structure of the complex of Cas1-Cas2 bound to the dual-forked DNA (PDB accession [[5dqz]]). The target DNA is shown in dark blue; the Cas1 and Cas2 dimers of the complex are indicated in blue and yellow, respectively. (B) Model explaining the capture of new DNA sequences from invading nucleic acid and the subsequent DNA integration into the host CRISPR array. The numbers on the left correspond to the order of events as described in the text. The dashed lines indicate nucleotides; the nucleotides C and N on the two sides of the protospacer are shown in red and green to clarify the orientation. From <ref name="Rev4">doi:10.1126/science.aad5147</ref>]] | ||
| + | {{Clear}} | ||
| + | |||
| + | In several type III CRISPR-Cas systems, Cas1 is fused to reverse transcriptase <ref name="Rev320">doi:10.1016/j.tibs.2009.05.002</ref>, and it was recently shown that these systems are capable of acquisition of RNA spacers by direct incorporation of an RNA segment into the CRISPR array followed by reverse transcription and replacement of the RNA strand by DNA <ref name="Rev462">doi:10.1126/science.aad4234</ref>. Although the biological function of this process remains to be elucidated, these findings demonstrate remarkable versatility of adaptation pathways. Spacer acquisition (adaptation) in type I systems proceeds along two distinct paths: (i) naïve acquisition, which occurs during an initial infection, and (ii) primed acquisition, when the CRISPR contains a previously integrated spacer that is complementary to the invading DNA <ref name="Rev463">doi:10.1016/j.virol.2012.10.003</ref>. According to the proposed model, naïve spacer adaptation involves five steps (Fig. 3B): | ||
| + | 1) Fragmentation of (mainly) invasive nucleic acids by non-Cas systems [e.g., by RecBCD after stalling a replication fork, or by restriction enzymes (restriction-modification systems) <ref name="Rev456">doi:10.1038/nature14302</ref><ref name="Rev459">doi:10.1038/ncomms3087</ref>] or by CRISPR-associated nucleases <ref name="Rev449">doi:10.1371/journal.pone.0035888</ref>. Although this step may be non-essential, it probably enhances the efficiency of the overall process and its specificity toward invading DNA. | ||
| + | 2) Selection of DNA fragments for (proto) spacers by scanning for potential PAMs (after partial target unwinding) by one of the four Cas1 subunits of the Cas1-Cas2 complex <ref name="Rev464">doi:10.1093/nar/gku510</ref>. | ||
| + | 3) Measuring of the selected protospacer generating fragments of the correct size with 3′ hydroxyl groups by Cas1 nuclease. | ||
| + | 4) Nicking of both strands of the leaderproximal repeat of the CRISPR array at the 5′ ends through a direct nucleophilic attack by the generated 3′ OH groups, resulting in covalent links of each of the strands of the newly selected spacer to the single-stranded repeat ends. | ||
| + | 5) Second-strand synthesis and ligation of the repeat flanks by a non-Cas repair system <ref name="Rev446">doi:10.1038/nrmicro.2015.14</ref><ref name="Rev461">doi:10.1038/nature14237</ref>.<ref name="Rev4">doi:10.1126/science.aad5147</ref> | ||
| + | |||
Primed spacer adaptation so far has been demonstrated only in type I systems <ref name="Rev450">doi:10.1038/ncomms1937</ref><ref name="Rev465">doi:10.1093/nar/gkt1154</ref><ref name="Rev466">doi:10.1093/nar/gku527</ref>. This priming mechanism constitutes a positive feedback loop that facilitates the acquisition of new spacers from formerly encountered genetic elements <ref name="Rev467">doi:10.1073/pnas.1400071111</ref>. Priming can occur even with spacers that contain several mismatches, making them incompetent as guides for targeting the cognate foreign DNA <ref name="Rev467">doi:10.1073/pnas.1400071111</ref>. Based on PAM selection, functional spacers are preferentially acquired during naïve adaptation. This initial acquisition event triggers a rapid priming response after subsequent infections. Priming appears to be a major pathway of CRISPR adaptation, at least for some type I systems <ref name="Rev465">doi:10.1093/nar/gkt1154</ref>. Primed adaptation strongly depends on the spacer sequence <ref name="Rev468">doi:10.1093/nar/gkv1259</ref>, and the acquisition efficiency is highest in close proximity to the priming site. In addition, the orientation of newly inserted spacers indicates a strand bias, which is consistentwith the involvement of singlestranded adaption intermediates <ref name="Rev469">doi:10.1093/nar/gkv1261</ref>. According to one proposed model <ref name="Rev470">doi:10.1093/nar/gkv1213</ref>, replication forks in the invader’s DNA are blocked by the Cascade complex bound to the priming crRNA, enabling the RecG helicase and the Cas3 helicase/nuclease proteins to attack the DNA. The ends at the collapsed forks then could be targeted by RecBCD, which provides DNA fragments for new spacer generation <ref name="Rev470">doi:10.1093/nar/gkv1213</ref>. Given that the use of crRNA for priming has much less strict sequence requirements than direct targeting of the invading DNA, priming is a powerful strategy that might have evolved in the course of the host-parasite arms race to reduce the escape by viral mutants, to provide robust resistance against invading DNA, and to enhance self/nonself discrimination. Naïve as well as primed adaptation in the subtype I-F system of Pseudomonas aeruginosa CRISPR-Cas require both the adaptation and the effector module <ref name="Rev469">doi:10.1093/nar/gkv1261</ref>.<ref name="Rev4">doi:10.1126/science.aad5147</ref> | Primed spacer adaptation so far has been demonstrated only in type I systems <ref name="Rev450">doi:10.1038/ncomms1937</ref><ref name="Rev465">doi:10.1093/nar/gkt1154</ref><ref name="Rev466">doi:10.1093/nar/gku527</ref>. This priming mechanism constitutes a positive feedback loop that facilitates the acquisition of new spacers from formerly encountered genetic elements <ref name="Rev467">doi:10.1073/pnas.1400071111</ref>. Priming can occur even with spacers that contain several mismatches, making them incompetent as guides for targeting the cognate foreign DNA <ref name="Rev467">doi:10.1073/pnas.1400071111</ref>. Based on PAM selection, functional spacers are preferentially acquired during naïve adaptation. This initial acquisition event triggers a rapid priming response after subsequent infections. Priming appears to be a major pathway of CRISPR adaptation, at least for some type I systems <ref name="Rev465">doi:10.1093/nar/gkt1154</ref>. Primed adaptation strongly depends on the spacer sequence <ref name="Rev468">doi:10.1093/nar/gkv1259</ref>, and the acquisition efficiency is highest in close proximity to the priming site. In addition, the orientation of newly inserted spacers indicates a strand bias, which is consistentwith the involvement of singlestranded adaption intermediates <ref name="Rev469">doi:10.1093/nar/gkv1261</ref>. According to one proposed model <ref name="Rev470">doi:10.1093/nar/gkv1213</ref>, replication forks in the invader’s DNA are blocked by the Cascade complex bound to the priming crRNA, enabling the RecG helicase and the Cas3 helicase/nuclease proteins to attack the DNA. The ends at the collapsed forks then could be targeted by RecBCD, which provides DNA fragments for new spacer generation <ref name="Rev470">doi:10.1093/nar/gkv1213</ref>. Given that the use of crRNA for priming has much less strict sequence requirements than direct targeting of the invading DNA, priming is a powerful strategy that might have evolved in the course of the host-parasite arms race to reduce the escape by viral mutants, to provide robust resistance against invading DNA, and to enhance self/nonself discrimination. Naïve as well as primed adaptation in the subtype I-F system of Pseudomonas aeruginosa CRISPR-Cas require both the adaptation and the effector module <ref name="Rev469">doi:10.1093/nar/gkv1261</ref>.<ref name="Rev4">doi:10.1126/science.aad5147</ref> | ||
Revision as of 10:29, 18 December 2016
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Mohanraju P, Makarova KS, Zetsche B, Zhang F, Koonin EV, van der Oost J. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science. 2016 Aug 5;353(6299):aad5147. doi: 10.1126/science.aad5147. PMID:27493190 doi:http://dx.doi.org/10.1126/science.aad5147
- ↑ Stern A, Keren L, Wurtzel O, Amitai G, Sorek R. Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet. 2010 Aug;26(8):335-40. doi: 10.1016/j.tig.2010.05.008. Epub 2010, Jul 1. PMID:20598393 doi:http://dx.doi.org/10.1016/j.tig.2010.05.008
- ↑ 3.0 3.1 3.2 3.3 3.4 Yosef I, Goren MG, Qimron U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 2012 Jul;40(12):5569-76. doi: 10.1093/nar/gks216. Epub 2012, Mar 8. PMID:22402487 doi:http://dx.doi.org/10.1093/nar/gks216
- ↑ Wei Y, Terns RM, Terns MP. Cas9 function and host genome sampling in Type II-A CRISPR-Cas adaptation. Genes Dev. 2015 Feb 15;29(4):356-61. doi: 10.1101/gad.257550.114. PMID:25691466 doi:http://dx.doi.org/10.1101/gad.257550.114
- ↑ 5.0 5.1 5.2 Levy A, Goren MG, Yosef I, Auster O, Manor M, Amitai G, Edgar R, Qimron U, Sorek R. CRISPR adaptation biases explain preference for acquisition of foreign DNA. Nature. 2015 Apr 23;520(7548):505-10. doi: 10.1038/nature14302. Epub 2015 Apr 13. PMID:25874675 doi:http://dx.doi.org/10.1038/nature14302
- ↑ Erdmann S, Le Moine Bauer S, Garrett RA. Inter-viral conflicts that exploit host CRISPR immune systems of Sulfolobus. Mol Microbiol. 2014 Mar;91(5):900-17. doi: 10.1111/mmi.12503. Epub 2014 Jan 17. PMID:24433295 doi:http://dx.doi.org/10.1111/mmi.12503
- ↑ Wigley DB. RecBCD: the supercar of DNA repair. Cell. 2007 Nov 16;131(4):651-3. PMID:18022359 doi:http://dx.doi.org/10.1016/j.cell.2007.11.004
- ↑ 8.0 8.1 Dupuis ME, Villion M, Magadan AH, Moineau S. CRISPR-Cas and restriction-modification systems are compatible and increase phage resistance. Nat Commun. 2013;4:2087. doi: 10.1038/ncomms3087. PMID:23820428 doi:http://dx.doi.org/10.1038/ncomms3087
- ↑ 9.0 9.1 Swarts DC, Mosterd C, van Passel MW, Brouns SJ. CRISPR interference directs strand specific spacer acquisition. PLoS One. 2012;7(4):e35888. doi: 10.1371/journal.pone.0035888. Epub 2012 Apr 27. PMID:22558257 doi:http://dx.doi.org/10.1371/journal.pone.0035888
- ↑ Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJ, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015 Nov;13(11):722-36. doi: 10.1038/nrmicro3569. Epub 2015, Sep 28. PMID:26411297 doi:http://dx.doi.org/10.1038/nrmicro3569
- ↑ 11.0 11.1 Nunez JK, Kranzusch PJ, Noeske J, Wright AV, Davies CW, Doudna JA. Cas1-Cas2 complex formation mediates spacer acquisition during CRISPR-Cas adaptive immunity. Nat Struct Mol Biol. 2014 May 4. doi: 10.1038/nsmb.2820. PMID:24793649 doi:http://dx.doi.org/10.1038/nsmb.2820
- ↑ Wang J, Li J, Zhao H, Sheng G, Wang M, Yin M, Wang Y. Structural and Mechanistic Basis of PAM-Dependent Spacer Acquisition in CRISPR-Cas Systems. Cell. 2015 Nov 5;163(4):840-53. doi: 10.1016/j.cell.2015.10.008. Epub 2015 Oct, 17. PMID:26478180 doi:http://dx.doi.org/10.1016/j.cell.2015.10.008
- ↑ 13.0 13.1 Nunez JK, Lee AS, Engelman A, Doudna JA. Integrase-mediated spacer acquisition during CRISPR-Cas adaptive immunity. Nature. 2015 Mar 12;519(7542):193-8. doi: 10.1038/nature14237. Epub 2015 Feb 18. PMID:25707795 doi:http://dx.doi.org/10.1038/nature14237
- ↑ van der Oost J, Jore MM, Westra ER, Lundgren M, Brouns SJ. CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem Sci. 2009 Aug;34(8):401-7. doi: 10.1016/j.tibs.2009.05.002. Epub, 2009 Jul 29. PMID:19646880 doi:http://dx.doi.org/10.1016/j.tibs.2009.05.002
- ↑ Silas S, Mohr G, Sidote DJ, Markham LM, Sanchez-Amat A, Bhaya D, Lambowitz AM, Fire AZ. Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase-Cas1 fusion protein. Science. 2016 Feb 26;351(6276):aad4234. doi: 10.1126/science.aad4234. PMID:26917774 doi:http://dx.doi.org/10.1126/science.aad4234
- ↑ Fineran PC, Charpentier E. Memory of viral infections by CRISPR-Cas adaptive immune systems: acquisition of new information. Virology. 2012 Dec 20;434(2):202-9. doi: 10.1016/j.virol.2012.10.003. Epub 2012, Nov 2. PMID:23123013 doi:http://dx.doi.org/10.1016/j.virol.2012.10.003
- ↑ Arslan Z, Hermanns V, Wurm R, Wagner R, Pul U. Detection and characterization of spacer integration intermediates in type I-E CRISPR-Cas system. Nucleic Acids Res. 2014 Jul;42(12):7884-93. doi: 10.1093/nar/gku510. Epub 2014, Jun 11. PMID:24920831 doi:http://dx.doi.org/10.1093/nar/gku510
- ↑ Amitai G, Sorek R. CRISPR-Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol. 2016 Feb;14(2):67-76. doi: 10.1038/nrmicro.2015.14. Epub 2016 , Jan 11. PMID:26751509 doi:http://dx.doi.org/10.1038/nrmicro.2015.14
- ↑ Datsenko KA, Pougach K, Tikhonov A, Wanner BL, Severinov K, Semenova E. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat Commun. 2012 Jul 10;3:945. doi: 10.1038/ncomms1937. PMID:22781758 doi:http://dx.doi.org/10.1038/ncomms1937
- ↑ 20.0 20.1 20.2 Li M, Wang R, Zhao D, Xiang H. Adaptation of the Haloarcula hispanica CRISPR-Cas system to a purified virus strictly requires a priming process. Nucleic Acids Res. 2014 Feb;42(4):2483-92. doi: 10.1093/nar/gkt1154. Epub 2013, Nov 21. PMID:24265226 doi:http://dx.doi.org/10.1093/nar/gkt1154
- ↑ Richter C, Dy RL, McKenzie RE, Watson BN, Taylor C, Chang JT, McNeil MB, Staals RH, Fineran PC. Priming in the Type I-F CRISPR-Cas system triggers strand-independent spacer acquisition, bi-directionally from the primed protospacer. Nucleic Acids Res. 2014 Jul;42(13):8516-26. doi: 10.1093/nar/gku527. Epub 2014, Jul 2. PMID:24990370 doi:http://dx.doi.org/10.1093/nar/gku527
- ↑ 22.0 22.1 Fineran PC, Gerritzen MJ, Suarez-Diez M, Kunne T, Boekhorst J, van Hijum SA, Staals RH, Brouns SJ. Degenerate target sites mediate rapid primed CRISPR adaptation. Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):E1629-38. doi:, 10.1073/pnas.1400071111. Epub 2014 Apr 7. PMID:24711427 doi:http://dx.doi.org/10.1073/pnas.1400071111
- ↑ Xue C, Seetharam AS, Musharova O, Severinov K, Brouns SJ, Severin AJ, Sashital DG. CRISPR interference and priming varies with individual spacer sequences. Nucleic Acids Res. 2015 Dec 15;43(22):10831-47. doi: 10.1093/nar/gkv1259. Epub, 2015 Nov 19. PMID:26586800 doi:http://dx.doi.org/10.1093/nar/gkv1259
- ↑ 24.0 24.1 Vorontsova D, Datsenko KA, Medvedeva S, Bondy-Denomy J, Savitskaya EE, Pougach K, Logacheva M, Wiedenheft B, Davidson AR, Severinov K, Semenova E. Foreign DNA acquisition by the I-F CRISPR-Cas system requires all components of the interference machinery. Nucleic Acids Res. 2015 Dec 15;43(22):10848-60. doi: 10.1093/nar/gkv1261. Epub, 2015 Nov 19. PMID:26586803 doi:http://dx.doi.org/10.1093/nar/gkv1261
- ↑ 25.0 25.1 Ivancic-Bace I, Cass SD, Wearne SJ, Bolt EL. Different genome stability proteins underpin primed and naive adaptation in E. coli CRISPR-Cas immunity. Nucleic Acids Res. 2015 Dec 15;43(22):10821-30. doi: 10.1093/nar/gkv1213. Epub, 2015 Nov 17. PMID:26578567 doi:http://dx.doi.org/10.1093/nar/gkv1213
- ↑ Hochstrasser ML, Doudna JA. Cutting it close: CRISPR-associated endoribonuclease structure and function. Trends Biochem Sci. 2015 Jan;40(1):58-66. doi: 10.1016/j.tibs.2014.10.007. Epub, 2014 Nov 18. PMID:25468820 doi:http://dx.doi.org/10.1016/j.tibs.2014.10.007
Categories: Topic Page | Crispr | Crispr-associated | Endonuclease | Cas9 | Cas6