User:Charli Barbet/Sandbox
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1gri' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1gri' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | Grb2 protein: Growth Factor Receptor Bound Protein is a cytosolic protein made of 217 amino acids and weighing 25,206 Da. Ubiquitously present in the cell, this protein is involved in signal transduction and especially in the MAP kinase pathway. Grb2 interacts mainly with tyrosine kinase such as [http://www.uniprot.org/uniprot/P00533 EGFR] once it’s been activated by ligand binding. This specific binding leads to the recruitment of GEF (like [ | + | Grb2 protein: Growth Factor Receptor Bound Protein is a cytosolic protein made of 217 amino acids and weighing 25,206 Da. Ubiquitously present in the cell, this protein is involved in signal transduction and especially in the MAP kinase pathway. Grb2 interacts mainly with tyrosine kinase such as [http://www.uniprot.org/uniprot/P00533 EGFR] once it’s been activated by ligand binding. This specific binding leads to the recruitment of GEF (like [http://www.uniprot.org/uniprot/Q07889 SOS1]), stimulating the activation of other pathways. Several others interactions have been elucidated like the capacity of the protein to dimerise thus implicated in the growth of malignant cells. |
== Structure == | == Structure == | ||
| Line 25: | Line 25: | ||
== Function == | == Function == | ||
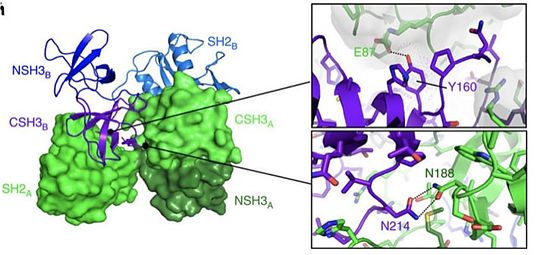
The Grb2 isoform has a non-functional SH2 domain, unable to link the phosphorylated tyrosine of its targeted protein (EGFR for instance). This inability of the molecule to transmit signal is traduce by apoptosis of the cell, thus regulating the growth signal. | The Grb2 isoform has a non-functional SH2 domain, unable to link the phosphorylated tyrosine of its targeted protein (EGFR for instance). This inability of the molecule to transmit signal is traduce by apoptosis of the cell, thus regulating the growth signal. | ||
| - | |||
The functional isoform Grb2 is involved in several cellular functions detailed below. On one hand, the SH2 domain recognizes phosphorylated residues which are mainly tyrosines. The recognized tyrosines present a caracteristic motif for recognition : NH2-pYXNX-COOH. | The functional isoform Grb2 is involved in several cellular functions detailed below. On one hand, the SH2 domain recognizes phosphorylated residues which are mainly tyrosines. The recognized tyrosines present a caracteristic motif for recognition : NH2-pYXNX-COOH. | ||
| Line 33: | Line 32: | ||
Thus by the special recognition of this motif, the binding of the 2 molecules is very specific. These motifs are highly expressed in several cellular proteins like Receptor Tyrosine Kinase (epidermal growth factor receptor, fibroblast growth factor receptor, nerve growth factor receptor) but equally in proteins that are not RTK kinases (BCR-Ab1, focal adhesion kinase, insulin receptor substrate-1). | Thus by the special recognition of this motif, the binding of the 2 molecules is very specific. These motifs are highly expressed in several cellular proteins like Receptor Tyrosine Kinase (epidermal growth factor receptor, fibroblast growth factor receptor, nerve growth factor receptor) but equally in proteins that are not RTK kinases (BCR-Ab1, focal adhesion kinase, insulin receptor substrate-1). | ||
| - | As an example, the SH2 domain of Grb2 recognizes an intracellular phosphorylated tyrosine. This binding, in turn, leads to the recruitment of [ | + | As an example, the SH2 domain of Grb2 recognizes an intracellular phosphorylated tyrosine. This binding, in turn, leads to the recruitment of [http://www.uniprot.org/uniprot/Q07889 SOS-1] via the SH3 domain of Grb2. Indeed, Grb2 is also made of two SH3 domains. These domains are able to recognize Proline rich region like the one of [http://www.uniprot.org/uniprot/Q07889 SOS-1] protein (Son Of Sevenless). |
| - | Following this pathway and the formation of a complex between Grb2 and [ | + | Following this pathway and the formation of a complex between Grb2 and [http://www.uniprot.org/uniprot/Q07889 SOS], the [http://www.uniprot.org/uniprot/P01112 RAS] protein is activated. Interestingly, [http://www.uniprot.org/uniprot/P01112 RAS] is a g-protein implicated in the activation of [http://www.uniprot.org/uniprot/P04049 RAF-1]. The latest activates of the MEK downstream cascade pathway ([http://www.uniprot.org/uniprot/Q02750 MEK1]/ [http://www.uniprot.org/uniprot/P36507 MEK2] et [http://www.uniprot.org/uniprot/P27361 ERK1 ]/ [http://www.uniprot.org/uniprot/P28482 ERK2]) involved in the translocation of ERK factors from the cytosol to the nucleus for the activation of [http://www.uniprot.org/uniprot/P19419 Elk-1] and [http://www.uniprot.org/uniprot/P01106 Myc transcription Factor]. These particular transcription factor participate in the activation of SRE containing gene leading to cellular growth. |
On the other hand, in T lymphocytes, the simulation of TCRs induces tyrosine phosphorylation on a wide range of of cellular proteins such as p36-38 or LAT. As an example, the phosphorylated residues of LAT can bind the SH2 domain of Grb2 while the formation of this complex recruits on the SH3 domain some proteins of the VAV family. VAV proteins are guanine nucleotide exchange factors (GEF) for the GTPase proteins of the Rho family. | On the other hand, in T lymphocytes, the simulation of TCRs induces tyrosine phosphorylation on a wide range of of cellular proteins such as p36-38 or LAT. As an example, the phosphorylated residues of LAT can bind the SH2 domain of Grb2 while the formation of this complex recruits on the SH3 domain some proteins of the VAV family. VAV proteins are guanine nucleotide exchange factors (GEF) for the GTPase proteins of the Rho family. | ||
This complex has for main aim to introduce a Calcium flux and the activation of MAP kinase allowing lymphocytes T proliferation. | This complex has for main aim to introduce a Calcium flux and the activation of MAP kinase allowing lymphocytes T proliferation. | ||
Revision as of 08:55, 13 January 2017
Grb2 (1gri)
| |||||||||||