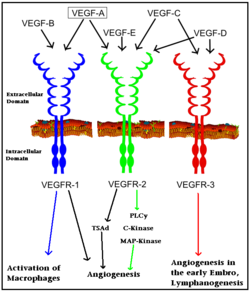
Vascular Endothelial Growth Factor Receptor
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
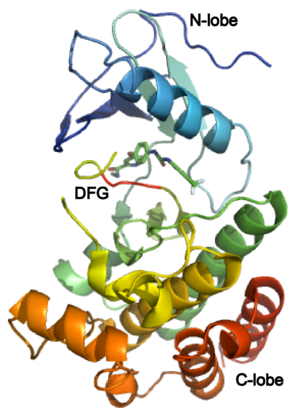
The structure of VEGFR-2 can been seen at the right. VEGF-A binds to the second and third extracellular Ig-like domains of VEGFR-2 with a 10-fold lower affinity than it does to the second Ig-like domain of VEGFR-1, despite the fact that VEGFR-2 is the principal mediator of several physiological effects on endothelial cells including proliferation, migration, and survival.<ref> PMID:9813036</ref> Binding of VEGF to the domains 2 and 3 of a VEGFR-2 monomer increases the probability that an additional VEGFR-2 binds the tethered ligand to form a dimmer. Once the two receptors are cross-linked, interactions between their membrane-proximal domain 7s stabilize the dimmer significantly. This dimerization and stabilization allows for precise positioning of the intracellular kinase domains, resulting in autophosphorylation and subsequent activation of the classical extracellular signal-regulated kinases (ERK) pathway.<ref>PMID:17293873</ref> | The structure of VEGFR-2 can been seen at the right. VEGF-A binds to the second and third extracellular Ig-like domains of VEGFR-2 with a 10-fold lower affinity than it does to the second Ig-like domain of VEGFR-1, despite the fact that VEGFR-2 is the principal mediator of several physiological effects on endothelial cells including proliferation, migration, and survival.<ref> PMID:9813036</ref> Binding of VEGF to the domains 2 and 3 of a VEGFR-2 monomer increases the probability that an additional VEGFR-2 binds the tethered ligand to form a dimmer. Once the two receptors are cross-linked, interactions between their membrane-proximal domain 7s stabilize the dimmer significantly. This dimerization and stabilization allows for precise positioning of the intracellular kinase domains, resulting in autophosphorylation and subsequent activation of the classical extracellular signal-regulated kinases (ERK) pathway.<ref>PMID:17293873</ref> | ||
| - | The tyrosine kinase domain of VEGFR-2 is separated into two segments with a 70 amino acid long kinase insert region. Upon binding VEGFA and subsequent dimerization, VEGFR-2 is autophosphoryalted at the carboxy terminal tail and kinase insert region. | + | The tyrosine kinase domain of VEGFR-2 is separated into two segments with a 70 amino acid long kinase insert region. Upon binding VEGFA and subsequent dimerization, VEGFR-2 is autophosphoryalted at the carboxy terminal tail and kinase insert region. '''FILL IN NEW MATERIAL.''' |
<br /> | <br /> | ||
Revision as of 06:23, 14 January 2017
| |||||||||||
3D Structures of VEGFR
Updated on 14-January-2017
Additional Resources
For additional information, see:
References
- ↑ Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359-71. PMID:16633338 doi:10.1038/nrm1911
- ↑ Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007 Oct;19(10):2003-12. Epub 2007 Jun 12. PMID:17658244 doi:10.1016/j.cellsig.2007.05.013
- ↑ Gallina P, Nohra G, Cioloca C, Meder JF, Roux FX. [Multiple cavernoma of delayed appearance] Neurochirurgie. 1994;40(5):322-5. PMID:7596453
- ↑ Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998 Nov 20;273(47):31283-8. PMID:9813036
- ↑ Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol. 2007 Mar;14(3):249-50. Epub 2007 Feb 11. PMID:17293873 doi:10.1038/nsmb1202
- ↑ Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002 Oct;2(10):795-803. PMID:12360282 doi:10.1038/nrc909
- ↑ Rosa DD, Ismael G, Lago LD, Awada A. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev. 2008 Feb;34(1):61-80. Epub 2007 Sep 10. PMID:17826917 doi:10.1016/j.ctrv.2007.07.019
- ↑ Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001 Sep;7(9):987-9. PMID:11533692 doi:10.1038/nm0901-987
- ↑ Rosa DD, Ismael G, Lago LD, Awada A. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev. 2008 Feb;34(1):61-80. Epub 2007 Sep 10. PMID:17826917 doi:10.1016/j.ctrv.2007.07.019
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Wayne Decatur