P53
From Proteopedia
(Difference between revisions)
| Line 40: | Line 40: | ||
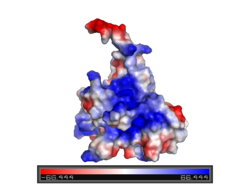
[[Image:P53_surface_charge.png | left | 250 px | thumb]]The figure at the left shows the surface charge of the p53 DNA-binding domain )Charged: red - negative; blue - positive). It is rich in arginine amino acids that interact with DNA, and this causes its surface to be positively charged. This domain recognizes specific regulatory sites on the DNA. The flexible structure of p53 allows it to bind to many different variants of binding sites, allowing it to regulate transcription at many places in the genome. | [[Image:P53_surface_charge.png | left | 250 px | thumb]]The figure at the left shows the surface charge of the p53 DNA-binding domain )Charged: red - negative; blue - positive). It is rich in arginine amino acids that interact with DNA, and this causes its surface to be positively charged. This domain recognizes specific regulatory sites on the DNA. The flexible structure of p53 allows it to bind to many different variants of binding sites, allowing it to regulate transcription at many places in the genome. | ||
| - | |||
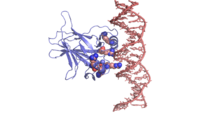
| - | <scene name='Sandbox/P53_dna_binding_domain/1'>Click Here to view a Three-dimensional Representation of the DNA-binding Domain Bound to DNA</scene> | ||
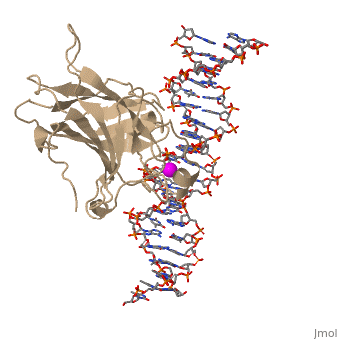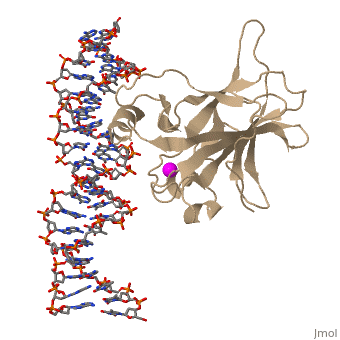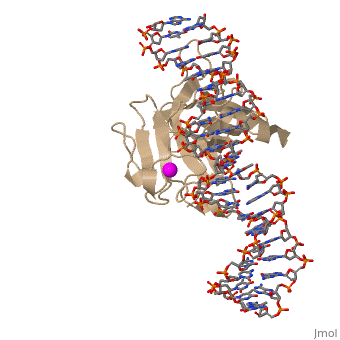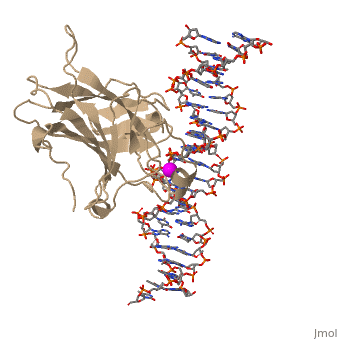
There is a Zn-binding motif on p53. The p53 Zn atom is coordinated by residues | There is a Zn-binding motif on p53. The p53 Zn atom is coordinated by residues | ||
Revision as of 15:57, 14 January 2017
| |||||||||||
3D structures of p53 (Updated on 14-January-2017)
Additional Resources
References
- ↑ Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003 Apr;10(4):431-42. PMID:12719720 doi:http://dx.doi.org/10.1038/sj.cdd.4401183
- ↑ Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a001107. doi:, 10.1101/cshperspect.a001107. PMID:20182618 doi:http://dx.doi.org/10.1101/cshperspect.a001107
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Michal Harel, Eran Hodis, Mary Ball, Alexander Berchansky, David Canner