Finasteride
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
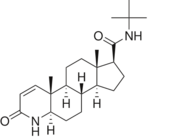
[[Image: Finasteride.PNG|thumb|180px|left|'''Fig. 1'''. Structure of Finasteride.]] | [[Image: Finasteride.PNG|thumb|180px|left|'''Fig. 1'''. Structure of Finasteride.]] | ||
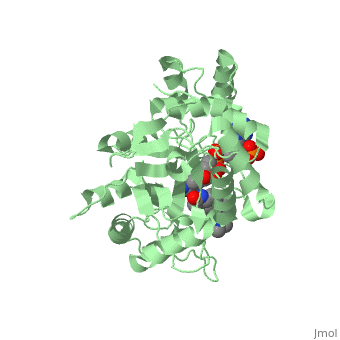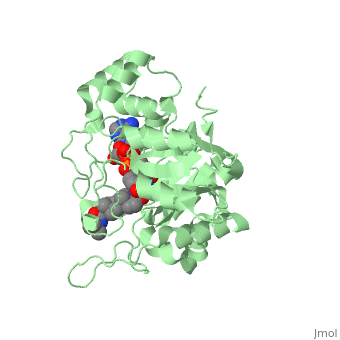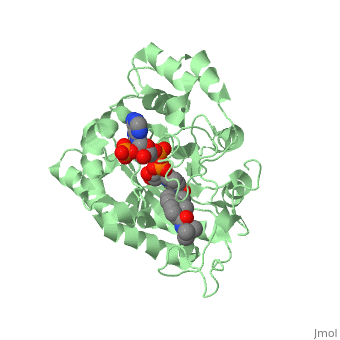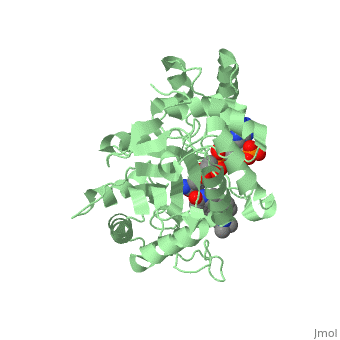
| - | [[Image: Capture.JPG|thumb|280px|right|'''Fig. 2'''The interaction between 5β-reductase (green) and Finasteride (gray) and NADP (blue). Two Tyrosine (58 and 132 in yellow), two Tryptophan (89 and 230 in red) and Glutamic acid (120 in orange) residues in 5β-reductase are interacting with Finasteride. While Glutamine (193 in light blue) and aspartic acid (53 in purple) residues in 5β-reductase are interacting with NADP.]] | + | [[Image: Capture.JPG|thumb|280px|right|'''Fig. 2''' The interaction between 5β-reductase (green) and Finasteride (gray) and NADP (blue). Two Tyrosine (58 and 132 in yellow), two Tryptophan (89 and 230 in red) and Glutamic acid (120 in orange) residues in 5β-reductase are interacting with Finasteride. While Glutamine (193 in light blue) and aspartic acid (53 in purple) residues in 5β-reductase are interacting with NADP.]] |
{{Clear}} | {{Clear}} | ||
Revision as of 21:12, 16 January 2017
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide (Finasteride)
| |||||||||||
References
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 2.2 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ 4.0 4.1 4.2 Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3
- ↑ Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9
- ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
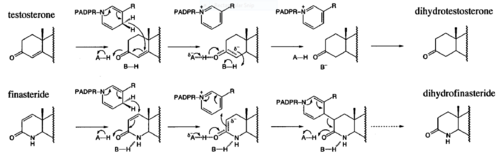
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
- ↑ Tacklind, J., Fink, H.A., MacDonald, R., Rutks, I., Wilt, T.J. (2010). Finasteride for benign prostatic hyperplasia. Cochrane database of systematic reviews 2010, Issue 10. Art. No.: CD006015. DOI: 10.1002/14651858.CD006015.pub3.
- ↑ Dragan, I., Misso, M. (2012). Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas, 72 (4), 269
- ↑ 10.0 10.1 Robaire, B., Henderson, N.A. (2006). Actions of 5alpha-reductase inhibitors on the epididymis. Molecular and Cellular Endocrinology. 250 (1-2): 190–5. doi:10.1016/j.mce.2005.12.044
- ↑ Paus, R., Cotsarelis, G. (1999).The biology of hair follicles. N. Engl. J. Med., 34, 491–497.
- ↑ Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December).
- ↑ Leyden, James et al.(June 1999)."Finasteride in the treatment of men with frontal male pattern hair loss." Journal of the American Academy of Dermatology. Volume 40 , Issue 6 , 930 - 937
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Cody J Cubbage, Michal Harel, Alexander Berchansky