User:Charli Barbet/Sandbox
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
Several others interactions have been elucidated like the capacity of the protein to dimerise proving its potential implication in the growth of [https://en.wikipedia.org/wiki/Malignancy malignant cells]. | Several others interactions have been elucidated like the capacity of the protein to dimerise proving its potential implication in the growth of [https://en.wikipedia.org/wiki/Malignancy malignant cells]. | ||
| - | == Structure == | + | == '''Structure''' == |
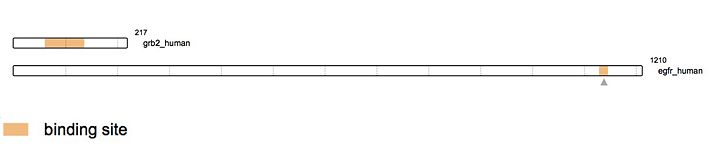
Grb2 protein has a very well characterized structure. Composed of 217 amino acids organized in two chains structured of [https://en.wikipedia.org/wiki/Beta_sheet β sheets] and [https://en.wikipedia.org/wiki/Alpha_helix α helices]. | Grb2 protein has a very well characterized structure. Composed of 217 amino acids organized in two chains structured of [https://en.wikipedia.org/wiki/Beta_sheet β sheets] and [https://en.wikipedia.org/wiki/Alpha_helix α helices]. | ||
[[Image:linearstructure.jpg|thumb|upright=5|[http://www.uniprot.org/uniprot/P62993#structure source]]] | [[Image:linearstructure.jpg|thumb|upright=5|[http://www.uniprot.org/uniprot/P62993#structure source]]] | ||
| Line 25: | Line 25: | ||
Grb3.3 is present in cells but it '''induces apoptosis'''. The isoform has a very similar structure to Grb2 but is truncated from an SH3 domain (from the 60th to the 100th amino) resulting in a degradation of its SH2 domain and a loss of functionality. <ref>PMID: 8178156</ref> | Grb3.3 is present in cells but it '''induces apoptosis'''. The isoform has a very similar structure to Grb2 but is truncated from an SH3 domain (from the 60th to the 100th amino) resulting in a degradation of its SH2 domain and a loss of functionality. <ref>PMID: 8178156</ref> | ||
| - | == Function == | + | == '''Function''' == |
The Grb2 isoform has a non-functional SH2 domain, unable to bind the phosphorylated tyrosine of its targeted protein (EGFR for instance). The inability of the molecule to transmit signal is translated by apoptosis of the cell, thus regulating growth signal. | The Grb2 isoform has a non-functional SH2 domain, unable to bind the phosphorylated tyrosine of its targeted protein (EGFR for instance). The inability of the molecule to transmit signal is translated by apoptosis of the cell, thus regulating growth signal. | ||
| Line 46: | Line 46: | ||
| - | == Interactions == | + | == '''Interactions''' == |
[http://www.uniprot.org/uniprot/Q07889 '''Sos1''']: Promotes the exchange of [http://www.uniprot.org/uniprot/P01112 Ras]-bound [https://en.wikipedia.org/wiki/Guanosine_diphosphate GDP] into [https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP], by promoting the [http://www.uniprot.org/uniprot/P01112 Ras] specific guanine nucleotide exchange factor activity. <ref>PMID: 10570290</ref> | [http://www.uniprot.org/uniprot/Q07889 '''Sos1''']: Promotes the exchange of [http://www.uniprot.org/uniprot/P01112 Ras]-bound [https://en.wikipedia.org/wiki/Guanosine_diphosphate GDP] into [https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP], by promoting the [http://www.uniprot.org/uniprot/P01112 Ras] specific guanine nucleotide exchange factor activity. <ref>PMID: 10570290</ref> | ||
| Line 68: | Line 68: | ||
| - | == EGFR interaction == | + | == '''EGFR interaction''' == |
[[Image:EGFR Grb2.jpg|thumb|upright=4|[http://www.ebi.ac.uk/intact/interaction/EBI-7874813 source]]] | [[Image:EGFR Grb2.jpg|thumb|upright=4|[http://www.ebi.ac.uk/intact/interaction/EBI-7874813 source]]] | ||
As stated earlier, Grb2 is made of an SH2 domain able to bind to [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase tyrosine kinase receptors]. Thus, Grb2 is able to bind to the activated form of the Epidermal Growth Factor Receptor ([http://www.uniprot.org/uniprot/P00533 EGFR]). [http://www.uniprot.org/uniprot/P00533 EGFR] activation mainly comes from the binding of a ligand. There is a wide range of ligands that are able to bind [http://www.uniprot.org/uniprot/P00533 EGFR] yet, the majority of the ligands come from the [https://en.wikipedia.org/wiki/ErbB ErbB family]. The most known ligands are [http://www.uniprot.org/uniprot/P01137 '''TGF-β'''] and [http://www.uniprot.org/uniprot/P01133 '''EGF''']. The binding of these latest induces [http://www.uniprot.org/uniprot/P00533 EGFR] dimerization. This '''dimerization''' activates the intracellular tyrosine kinase domain characterized by the '''autophosphorylation of tyrosines (Y992, Y1045, Y1068, Y1086 and Y1173'''). The activated form of [http://www.uniprot.org/uniprot/P00533 EGFR] then recruits Grb2. Indeed, the SH2 domain of Grb2 (from the 60th to the 152nd amino acid) binds the phosphorylated tyrosines of [http://www.uniprot.org/uniprot/P00533 EGFR] (Y1068 & Y1086). This interaction recruits [http://www.uniprot.org/uniprot/Q07889 SOS] (Son Of Sevenless) via the SH3 domain of Grb2. [http://www.uniprot.org/uniprot/Q07889 SOS] is a [https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor GEF protein] activating [http://www.uniprot.org/uniprot/P01112 RAS] and therefore in turn the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK pathway]. <ref>PMID: 16273093</ref> | As stated earlier, Grb2 is made of an SH2 domain able to bind to [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase tyrosine kinase receptors]. Thus, Grb2 is able to bind to the activated form of the Epidermal Growth Factor Receptor ([http://www.uniprot.org/uniprot/P00533 EGFR]). [http://www.uniprot.org/uniprot/P00533 EGFR] activation mainly comes from the binding of a ligand. There is a wide range of ligands that are able to bind [http://www.uniprot.org/uniprot/P00533 EGFR] yet, the majority of the ligands come from the [https://en.wikipedia.org/wiki/ErbB ErbB family]. The most known ligands are [http://www.uniprot.org/uniprot/P01137 '''TGF-β'''] and [http://www.uniprot.org/uniprot/P01133 '''EGF''']. The binding of these latest induces [http://www.uniprot.org/uniprot/P00533 EGFR] dimerization. This '''dimerization''' activates the intracellular tyrosine kinase domain characterized by the '''autophosphorylation of tyrosines (Y992, Y1045, Y1068, Y1086 and Y1173'''). The activated form of [http://www.uniprot.org/uniprot/P00533 EGFR] then recruits Grb2. Indeed, the SH2 domain of Grb2 (from the 60th to the 152nd amino acid) binds the phosphorylated tyrosines of [http://www.uniprot.org/uniprot/P00533 EGFR] (Y1068 & Y1086). This interaction recruits [http://www.uniprot.org/uniprot/Q07889 SOS] (Son Of Sevenless) via the SH3 domain of Grb2. [http://www.uniprot.org/uniprot/Q07889 SOS] is a [https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor GEF protein] activating [http://www.uniprot.org/uniprot/P01112 RAS] and therefore in turn the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK pathway]. <ref>PMID: 16273093</ref> | ||
| Line 75: | Line 75: | ||
<br style="clear:both" /> | <br style="clear:both" /> | ||
| - | == Diseases == | + | == '''Diseases''' == |
[https://en.wikipedia.org/wiki/Alzheimer's_disease '''Alzheimer’s Disease (AD)''']: | [https://en.wikipedia.org/wiki/Alzheimer's_disease '''Alzheimer’s Disease (AD)''']: | ||
| Line 86: | Line 86: | ||
Grb2's isoform could have '''a simulatory effect in the retro viral infection of''' [https://en.wikipedia.org/wiki/Alzheimer's_disease '''HIV-1''']. By its essential role in the MAPK pathway, Grb3 can have effects on [https://en.wikipedia.org/wiki/Alzheimer's_disease HIV-1] infections. Indeed, the replication of the virus is activated by [https://en.wikipedia.org/wiki/T_cell Lymphocytes T] replication. Yet [https://en.wikipedia.org/wiki/T_cell Lymphocytes T]’s activation depends on the activation of the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK pathway] dictated by the presence or not of Grb3 in the cell. This pathway finally activates [http://www.uniprot.org/uniprot/Q14934 NFAT], a transcription factor enhancing the [https://en.wikipedia.org/wiki/Long_terminal_repeat LTR promotor] of [https://en.wikipedia.org/wiki/Alzheimer's_disease HIV-1] leading to its replication. <ref>PMID: 10906142</ref> | Grb2's isoform could have '''a simulatory effect in the retro viral infection of''' [https://en.wikipedia.org/wiki/Alzheimer's_disease '''HIV-1''']. By its essential role in the MAPK pathway, Grb3 can have effects on [https://en.wikipedia.org/wiki/Alzheimer's_disease HIV-1] infections. Indeed, the replication of the virus is activated by [https://en.wikipedia.org/wiki/T_cell Lymphocytes T] replication. Yet [https://en.wikipedia.org/wiki/T_cell Lymphocytes T]’s activation depends on the activation of the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK pathway] dictated by the presence or not of Grb3 in the cell. This pathway finally activates [http://www.uniprot.org/uniprot/Q14934 NFAT], a transcription factor enhancing the [https://en.wikipedia.org/wiki/Long_terminal_repeat LTR promotor] of [https://en.wikipedia.org/wiki/Alzheimer's_disease HIV-1] leading to its replication. <ref>PMID: 10906142</ref> | ||
| - | == Relevance == | + | == '''Relevance''' == |
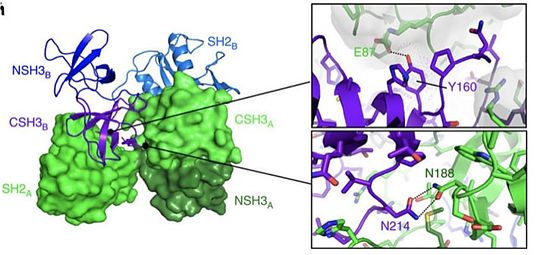
[[Image:Y160.jpg|thumb|upright=3|[http://www.nature.com/articles/ncomms8354#abstract source]]] | [[Image:Y160.jpg|thumb|upright=3|[http://www.nature.com/articles/ncomms8354#abstract source]]] | ||
| Line 93: | Line 93: | ||
<br style="clear:both" /> | <br style="clear:both" /> | ||
| - | == References == | + | == '''References''' == |
<references/> | <references/> | ||
Revision as of 11:46, 23 January 2017
Grb2 (1gri)
| |||||||||||