We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Estelle Metzger/Sandbox
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
Thanks to the similarity between LRRK2 and Roco4 from the ''Dictyostelium'', Roco4 is used in studies with a view to finding that inhibitor. One of the candidates to inhibit this activity is LRRK2-IN-1<ref name="Bernd">doi: 10.1021/jm5018779</ref>. | Thanks to the similarity between LRRK2 and Roco4 from the ''Dictyostelium'', Roco4 is used in studies with a view to finding that inhibitor. One of the candidates to inhibit this activity is LRRK2-IN-1<ref name="Bernd">doi: 10.1021/jm5018779</ref>. | ||
| - | Pour ajouter une référence : <ref>Rentrer doi:... de la publi</ref> et si on veut utiliser la réf plusieurs fois, il faut lui donner un nom | ||
== Humanized Roco4 == | == Humanized Roco4 == | ||
| Line 28: | Line 27: | ||
== LRRK2-IN-1 == | == LRRK2-IN-1 == | ||
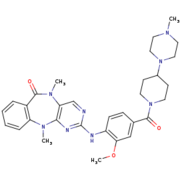
| - | + | [[Image:4K4-270.png|thumb|LRRK2-IN-1 structure]] | |
| - | <scene name='75/751216/Lrrk2-in-1/1'>LRRK2-IN-1</scene> is a type 1 inhibitor. It is the first identified LRRK2-specific inhibitor, which is now a common tool compound for the LRRK2 research community. LRRK2-IN-1 has a 2-amino-5,11- dimethyl-5H-benzo[e]pyrimido[5,4-b][1,4]diazepine-6(11H)-one scaffold. | + | <scene name='75/751216/Lrrk2-in-1/1'>LRRK2-IN-1</scene> is a type 1 inhibitor. It is the first identified LRRK2-specific inhibitor, which is now a common tool compound for the LRRK2 research community. LRRK2-IN-1 has a 2-amino-5,11- dimethyl-5H-benzo[e]pyrimido[5,4-b][1,4]diazepine-6(11H)-one scaffold. |
The function is of LRRK2-In-1 is to dephosphorylate LRRK2 residues Ser910 and Ser935 in the kidney, but not in the brain. This compound is not capable of crossing the blood-brain barrier. | The function is of LRRK2-In-1 is to dephosphorylate LRRK2 residues Ser910 and Ser935 in the kidney, but not in the brain. This compound is not capable of crossing the blood-brain barrier. | ||
The structure of LRRK2-In-1 does not stabilize the active conformation. Indeed, the activation loop is poorly resolved indicating that it is flexible. Moreover, it presents a closure of the glycine-rich loop in the inhibitor structure <ref name="Bernd"/>. | The structure of LRRK2-In-1 does not stabilize the active conformation. Indeed, the activation loop is poorly resolved indicating that it is flexible. Moreover, it presents a closure of the glycine-rich loop in the inhibitor structure <ref name="Bernd"/>. | ||
Revision as of 15:14, 26 January 2017
Humanized Roco4 bound to LRRK2-IN-1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Gilsbach BK, Messias AC, Ito G, Sattler M, Alessi DR, Wittinghofer A, Kortholt A. Structural Characterization of LRRK2 Inhibitors. J Med Chem. 2015 May 1. PMID:25897865 doi:http://dx.doi.org/10.1021/jm5018779
- ↑ Gilsbach BK, Kortholt A. Structural biology of the LRRK2 GTPase and kinase domains: implications for regulation. Front Mol Neurosci. 2014 May 5;7:32. doi: 10.3389/fnmol.2014.00032. eCollection, 2014. PMID:24847205 doi:http://dx.doi.org/10.3389/fnmol.2014.00032