User:Ophelie Lefort/Sandbox
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
== Structural highlights == | == Structural highlights == | ||
| - | |||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
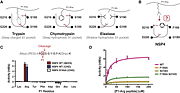
The protein has a 283 amino-acids long sequence with two mainly domains [[Image:Linear structure of PRSS57.png | thumb]]. It forms a kind of elongate sphere of approximately 70Å large and 150Å long (dimensions: 70,37Å X 70,37Å X 105,02Å). The protein is composed of signal peptide (1-31) which leads to the location in azurophil granules [http://www.jimmunol.org/content/191/5/2700] and allows its excretion. In addition there is a protease domain (32-283) which is a trypsin-like domain with a trypsin-like active site, according to the specificity for P1-Arg residues, but this domain can be an elastase-like active site according to the primary sequence (because of the presence of a swallow S1 pocket) specific to small aliphatic residues.<ref>S.. Jack Lin, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, Daniel Kirchhofer Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease DOI: http://dx.doi.org/10.1016/j.str.2014.07.008</ref> | The protein has a 283 amino-acids long sequence with two mainly domains [[Image:Linear structure of PRSS57.png | thumb]]. It forms a kind of elongate sphere of approximately 70Å large and 150Å long (dimensions: 70,37Å X 70,37Å X 105,02Å). The protein is composed of signal peptide (1-31) which leads to the location in azurophil granules [http://www.jimmunol.org/content/191/5/2700] and allows its excretion. In addition there is a protease domain (32-283) which is a trypsin-like domain with a trypsin-like active site, according to the specificity for P1-Arg residues, but this domain can be an elastase-like active site according to the primary sequence (because of the presence of a swallow S1 pocket) specific to small aliphatic residues.<ref>S.. Jack Lin, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, Daniel Kirchhofer Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease DOI: http://dx.doi.org/10.1016/j.str.2014.07.008</ref> | ||
Revision as of 18:35, 26 January 2017
link title
|
| |||||||||||
References
[1] NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity, NCBI
[2] NSP4, an elastase-related protease in human neutrophils with arginine specificity, NCBI
[3] Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response, NCBI
[4] Role of neutrophils in innate immunity: a systems biology-level approach, NCBI
[5] Neutrophils: Their Role in Innate and Adaptive Immunity, NCBI
[6] Neutrophil extracellular traps kill bacteria, NCBI
[7] Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response, NCBI
- ↑ S.. Jack Lin, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, Daniel Kirchhofer Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease DOI: http://dx.doi.org/10.1016/j.str.2014.07.008
- ↑ Natascha C. Perera, Karl-Heinz Wiesmüller, Maria Torp Larsen, Beate Schacher, Peter Eickholz, Niels Borregaard and Dieter E. Jenne NSP4 Is Stored in Azurophil Granules and Released by Activated Neutrophils as Active Endoprotease with Restricted Specificity DOI: https://doi.org/10.4049/jimmunol.1301293