Elizeu/sandbox/citocromo c
From Proteopedia
| Line 3: | Line 3: | ||
'''Uniprot Accesion:''' POA4G2 | '''Uniprot Accesion:''' POA4G2 | ||
| - | '''PDB ID''': | + | '''PDB ID''': 3H90 |
| - | < | + | <Structure load='3H90' size='350' frame='true' align='right' caption='3D image of structural transporter Yiip'/> |
| - | == Overview == | ||
| - | '''[[Image:streptococcus pneumoniae.jpg | 250px|thumb| ''Streptococcus pneumoniae'' <ref>http://www.sciencephoto.com/images/download_lo_res.html?id=662360183</ref>]] PsaA (Pneumococcal surface antigen A) is a high affinity substrate-binding lipoprotein <ref name="ref5">http://www.uniprot.org/uniprot/P0A4G2</ref> detected on all known serotypes of [https://en.wikipedia.org/wiki/Streptococcus_pneumoniae ''Streptococcus pneumonia'']. It facilitates the acquisition of Mn<sup>2+</sup> by being part of the cell's [https://de.wikipedia.org/wiki/ABC-Transporter ABC (ATP binding cassette) transporter] and plays a major role in pneumococcal attachment to the host cell and [https://en.wikipedia.org/wiki/Virulence virulence] <ref>PMID:25786639</ref>.''' | ||
| - | + | == Overview == | |
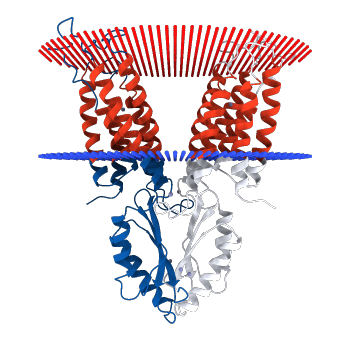
| - | + | '''[[Image:3h90.0 chimera tm 350 350.png]] Structural Transporter Yiip | |
| - | === | + | |
| - | + | ===General Structure=== | |
| + | yiip has two dimers, connected by salt bridges in the alpha helices | ||
| - | In the case of [https://www.wikigenes.org/e/gene/e/2717000.html PsaA], the ABC transporter is composed of an ATP-binding protein (''PsaB''), an integral membrane protein (''PsaC'') and PsaA itself as a [https://en.wikipedia.org/wiki/Lipoprotein lipoprotein] that possess a metal-ion binding site. This confers the protein to bind divalent metal-ions, however, with a preference for Mn<sup>2+</sup> <ref>PMID:18340341</ref>. | ||
| - | The genes that encode for the components of the ABC transport system are ''psaA'', ''psaB'' and ''psaC'', respectively. They are organized consecutively in the psaBCA operon, together with ''psaD'', the gene encoding for a [[thiol peroxidase]] (see below). <ref name="ref3">PMID:517531</ref>. | ||
| - | [[Image:operon psa.jpg |600px|center| thumb| PsaBCA operon <ref>PMID:15255900</ref>]] | ||
| - | == | + | == Interaction of Dimers == |
| + | |||
| + | the dimers interact via salt bridges and electrostatic interactions | ||
| + | |||
| + | |||
| + | |||
| + | === Salt Bridges === | ||
| - | + | insert photo and explanation here | |
| + | === Electrostatic Interactions === | ||
| - | == General Structure == | ||
| - | The protein PsaA has a molecular weight of 34.538 kDa with 309 residues <ref>https://www.mybiosource.com/prods/Recombinant-Protein/Manganese-ABC-transporter-substrate-binding-lipoprotein-psaA/psaA</ref>. The overall size of the protein approximated from its crystal structure is 40 by 40 by 70 Å <ref name="ref1">PMID:28011228</ref>. | ||
| - | As a member of the Lipoprotein receptor-associated antigen I (LraI) family, the PsaA molecule contains four distinct regions. An N-terminal leader sequence of 20 amino acids holds an LxACy consensus sequence that is recognized and cleaved by signal peptidase II <ref name="ref1" />. A lipid moiety (diacylglycerol <ref name="ref2">PMID:99024</ref>) is added to the cysteine residue and mediates the anchorage of the protein to the cytoplasmic membrane. Apart from this leader sequence, the rest of the protein consists of two twofold-pseudosymmetrical (β/α)<sub>4</sub> sandwich domains, of which the β-strands of each domain form parallel β-sheets <ref name="ref2" />. In total these domains form two lobes connected via an α-helical linker which constitutes the solute-binding site <ref name="ref1" />. | ||
| - | '''Secondary structure of PsaA:''' | ||
| - | [[Image:structure secondaire.jpg |1000 px|center| thumb| Secondary structure of PsaA <ref name="ref5" />]] | ||
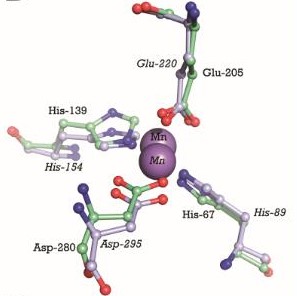
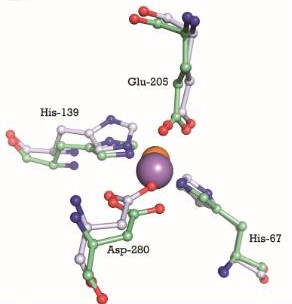
| - | We can see on the right, the 3D structure of PsaA protein. We can observe that there are <scene name='55/559112/3zk7_tris/1'>TRIS molecules</scene>(TRIS significate 2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL). | ||
| - | We can see the <scene name='55/559112/3ztt/1'>3D structure of PsaA with manganese</scene>. Manganese ions are shown as | ||
| - | <scene name='55/559112/3ztt_purple_sphere/1'>purple spheres</scene>. | ||
| - | {| | ||
| - | | [[Image:molec.jpg|left|300px | thumb| 3D structure of PsaA with 2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL (tris) and cadmium <ref>http://www.ebi.ac.uk/pdbe/entry/pdb/4UTO</ref>]] | ||
| - | | [[Image:showNew.jpg| 250px| thumb| Structure of Tris PsaA needs to be linked to this molecule to be in an open state]] | ||
| - | |} | ||
| - | + | <Structure load='Insert PDB code or filename here' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | |
Cadmium uptake reduces the millimolar cellular accumulation of manganese and zinc, and thereby increases sensitivity to oxidative stress <ref>http://www.nature.com/articles/ncomms7418</ref>. | Cadmium uptake reduces the millimolar cellular accumulation of manganese and zinc, and thereby increases sensitivity to oxidative stress <ref>http://www.nature.com/articles/ncomms7418</ref>. | ||
Revision as of 09:44, 2 March 2017
NCBI Accession: P42363.1
Uniprot Accesion: POA4G2
PDB ID: 3H90
|
Contents |
Overview
General Structure
yiip has two dimers, connected by salt bridges in the alpha helices
Interaction of Dimers
the dimers interact via salt bridges and electrostatic interactions
Salt Bridges
insert photo and explanation here
Electrostatic Interactions
|
Cadmium uptake reduces the millimolar cellular accumulation of manganese and zinc, and thereby increases sensitivity to oxidative stress [1].
| 
|
Function
Mn2+ enables Streptococcus Pneunomiae to have a prompt and rapid growth. Superoxides are a key player in oxidative stress generated in the presence of iron. Therefore PsaA is essential for the existence of S. pneumoniae as mutations in the psaA gene cause deficiencies in growth but also in its virulence, adherence and the response to oxidative stress.
Adhesin and virulence
PsaA is well hidden beneath the cell wall and the polysaccharide capsule, which is why it does not directly act as an adhesin as initially assumed. This was confirmed by studies on psaB and psaC deletion mutants which were not deficient in PsaA but showed impaired adherence, similar to psaA deletion mutants. As the disruption of either of the members of the Psa operon affects pneumococcal adherence, it seems that this process is coupled to the cellular Mn2+ transport system. With bacterial adhesion being the first step in bacterial pathogenesis, the extinction of PsaA and the associated Psa operon lead to attenuated virulence in affected strains [2].
Virulence
Next to its involvement in bacterial adhesion and the colonization in the human nasopharynx, S. pneumoniae actively produces H2O2 in large amounts to augment its own virulence on the one hand, and, on the other hand, to utilize it as a weapon in the competition against other pathogens [2]. Mn2+ is also required for the activity of CpsB, a tyrosine phosphatase involved in the regulation of capsule production. And in some streptococcal species, lectin-mediated adherence requires Mn2+. Finally, mutations in the psaBCA operon result in an almost complete attenuation of virulence for all tested models of animal infection.
Oxidative stress
In S. pneumoniae H2O2 and superoxides are generated as toxic derivatives of molecular oxygen in several metabolic processes and during growth by pyruvate oxidase. Subsequently, H2O2 reacts with Fe2+ during the Fenton reaction. This results in the formation of the hydroxylyl radical that causes severe damages in the cell. As a protective response against this oxidative stress, S. pneumoniae produces an Mn2+-dependent superoxide dismutase (SodA) and the thiol peroxidase encoded by psaD in the Psa operon. Due to the use of H2O2 during pneumococcal virulence these two proteins and the dependence on Mn2+ are of major importance. When Mn2+ transport is disrupted, bacterial cells display hypersensitivity towards oxidative stress [2].
Interactions with other proteins
- piaA, pneumococcal iron acquisition A which is an iron-compound ABC transporter permease. It is required for bacterial growth and full virulence in pulmonary infection.
-pavA, Pneumococcal adherence and virulence factor A is an adherence and virulence protein A. It binds fibronectin.
-Wzg, which regulates the capsule production and is an integral membrane regulatory protein Cps2A
-Tpx, a thiol peroxidase with a Has antioxidant activity. This protein can remove peroxides or H2O2 by similarity.
-PsaC, a manganese ABC transporter permease
-PsaB, a manganese ABC transporter ATP-binding protein. Deleting psaB results in decreased expression of PsaA. PsaC and psaC require Mn2+ supplementation for growth and transformation.
-SPD_1450, an iron-dependent transcriptional regulator
-adcC, antibody-dependent cell-mediated cytotoxicity is a zinc ABC transporter ATP-binding protein.
-adcB, a zinc ABC transporter permease
Disease
Otitis media
PsaA has been recognized to be involved in the adherence and virulence mechanisms of otitis media. Streptococcus pneumoniae is one of the main agents causing bacterial acute otitis media, directly or as complication of a viral upper respiratory tract infection. This disease is a highly prevalent pediatric disease worldwide. Hearing loss is a common problem associated with this disease. Streptococcus pneumoniae in middle ear can be resistant to penicillin and cephalosporin[3]
Pneumonia
PsaA protein is an indirect adhesin which is involved in colonization of the nasopharyngeal mucosal. Moreover, alveolar pneumonia is caused by the spread of Streptococcus pneumoniae from nasopharynx. Therefore PsaA protein is involved in infection of pulmonary parenchyma by Streptococcus pneumoniae. Sometimes, Streptococcus pneumoniae pass into the blood and causes a bacteremia besides pneumonia.
Application in Biotechnology
PsaA is being actively evaluated as a component of a vaccin in formulations composed of pneumococcal common proteins. PsaA is a component of a vaccin because this protein is immunogenic and stimulates an increase in antibody production when the nasopharynx is naturally colonized. PsaA has been expressed as an E.coli recombinant protein, purified, and evaluated in a phase one clinical trial. Progress in vaccine development is most advanced for Streptococcus pneumoniae. Indeed, there is a seven-valent capsular-conjugate vaccine, PREVNAR® but it is rather non efficient for otitis media.[4] PsaA has been shown to be an effective vaccine in animal models at preventing Streptococcus pneumoniae infection. <math>Insert formula here</math>
References
- ↑ http://www.nature.com/articles/ncomms7418
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedref3 - ↑ https://www.google.com/patents/US20130078254
- ↑ Wald ER, Mason EO Jr, Bradley JS, Barson WJ, Kaplan SL. Acute otitis media caused by Streptococcus pneumoniae in children's hospitals between 1994 and 1997. Pediatr Infect Dis J. 2001 Jan;20(1):34-9. PMID:11176564
Proteopedia Page Contributors and Editors (what is this?)
Julie Langlois, Atena Farhangian, Rebecca Holstein, Elizabeth A. Dunlap, Katherine Reynolds, Elizeu Santos, Noam Gonen, Anna Lohning, Idan Ben-Nachum, Brian Ochoa, Shai Biran, Gauri Misra, Shira Weingarten-Gabbay, Keni Vidilaseris, Jamie Costa, Abhinav Mittal, Urs Leisinger, Madison Walberry, Edmond R Atalla, Brett M. Thumm, Brooke Fenn, Joel L. Sussman, Mati Cohen, Vesta Nwankwo, Dotan Shaniv, Gulalai Shah