We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1067
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='3h90' size='350' side='right' caption='Escherichia coli reca protein-bound DNA (PDB entry [[3h90]])' scene=''> | <StructureSection load='3h90' size='350' side='right' caption='Escherichia coli reca protein-bound DNA (PDB entry [[3h90]])' scene=''> | ||
| - | <scene name='69/694234/3h90/ | + | <scene name='69/694234/3h90/2'>3h90</scene> is an integral membrane protein of the E. coli bacteria that regulates the import and export of Zn<sup>2+</sup>. |
== Structure == | == Structure == | ||
| Line 10: | Line 10: | ||
=== Electrostatic Interactions === | === Electrostatic Interactions === | ||
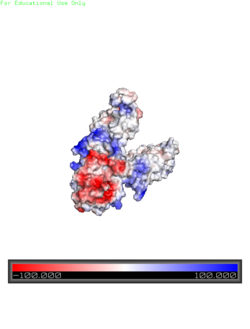
| - | [[Image:Yiip_Electrostatic.png|250px|left|thumb|Electrostatics]]<scene name='69/694234/ | + | [[Image:Yiip_Electrostatic.png|250px|left|thumb|Electrostatics]]<scene name='69/694234/Electro/1'>Charge distribution</scene> along the exterior surface of the protein is primarily neutral for the TMDs, but transitions to positive near the location of the charge interlock and interior side of the cell membrane. This positive section is characteristic of trans-membrane proteins as a means of achieving proper orientation. Binding sites A, B, and C, as well as the CTDs of both monomers, all possess a high negative charge relative to the other charges present, facilitating the binding and releasing of Zn<sup>2+</sup> ions. The two CTDs are held together by the charge interlock and hydrophobic interactions of the TMDs despite their electrostatic repulsion. Upon the release of Zn<sup>2+</sup> ions, the CTDs undergo electronegativity alterations, forcing the two domains apart. |
=== Conformation Changes === | === Conformation Changes === | ||
Revision as of 17:42, 24 March 2017
Zinc Transporter Yiip
| |||||||||||