Sandbox Reserved 1071
From Proteopedia
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
=DgcZ from ''E. coli''= | =DgcZ from ''E. coli''= | ||
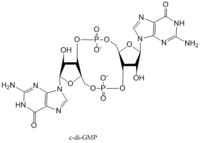
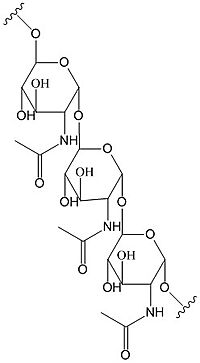
| - | Diguanylate cyclases synthesize cyclic dimeric-GMP (c-di-GMP) from two GTP molecules. [[Image:C-di-GMP.jpg |200 px| | + | Diguanylate cyclases synthesize cyclic dimeric-GMP (c-di-GMP) from two GTP molecules. [[Image:C-di-GMP.jpg |200 px|right|thumb|cyclic-dimeric-GMP]] C-di-GMP is a second messenger in the production of poly-β-1,6-N-acetylglucosamine (poly-GlcNAc) [[Image:Poly B-1,6 GlcNAc.jpg |200 px|right|thumb|poly-β-1,6-N-acetylglucosamine]] , a polysaccharide required for ''E. coli'' biofilm production. This biofilm allows ''E. coli'' to adhere to extracellular surfaces. The DgcZ protein is made of two domains: the catalytic GGDEF domain responsible for sythnesizing c-di-GMP and the regulatory CZB domain that binds zinc. When zinc is bound, the CZB and GGDEF domains adopt conformations that inhibit DgcZ function. DgcZ binds zinc with sub-femtomolar affinity, making it very likely that zinc will bind in the CZB domain. <StructureSection load='4h54' size='300' frame='true' align='right' caption='E. coli Diguanylate cyclase'> |
</StructureSection> | </StructureSection> | ||
| - | = | + | =Structure= |
| - | ==GGDEF Domain== | + | ==Catalytic GGDEF Domain== |
| + | ''E. coli'' DgcZ is a protein made of two domains each of which is a symmetric homodimer. The GGDEF domain is made of a central five-stranded β-sheet with five α-helices surrounding it. Each dimer contains an active half-site that, when combined together in a productive conformation, form the entire active site. Each half-site binds one GTP. The guanyl base forms hydrogen bonds with asparagine-173 and aspartate-182 to hold it in the active site. A magnesium<sup>2+</sup> ion stabilizes the negative charges on the phosphate groups. When in the productive conformation, each GTP is held in close proximity with the α-phosphate groups overlapping C3 of the ribose. This conformation allows the α-phospate of one GTP to react with alcohol on C3 on the ribose of the other GTP, resulting in a cyclization of the two molecules into cyclic-dimeric-GMP. | ||
| + | |||
| + | |||
==CZB Domain== | ==CZB Domain== | ||
| Line 11: | Line 14: | ||
= Zinc Ligand(s) = | = Zinc Ligand(s) = | ||
| + | ==Inhibition== | ||
= Other Ligands = | = Other Ligands = | ||
Revision as of 17:54, 24 March 2017
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Contents |
DgcZ from E. coli
Diguanylate cyclases synthesize cyclic dimeric-GMP (c-di-GMP) from two GTP molecules. C-di-GMP is a second messenger in the production of poly-β-1,6-N-acetylglucosamine (poly-GlcNAc) , a polysaccharide required for E. coli biofilm production. This biofilm allows E. coli to adhere to extracellular surfaces. The DgcZ protein is made of two domains: the catalytic GGDEF domain responsible for sythnesizing c-di-GMP and the regulatory CZB domain that binds zinc. When zinc is bound, the CZB and GGDEF domains adopt conformations that inhibit DgcZ function. DgcZ binds zinc with sub-femtomolar affinity, making it very likely that zinc will bind in the CZB domain.
| |||||||||||
Structure
Catalytic GGDEF Domain
E. coli DgcZ is a protein made of two domains each of which is a symmetric homodimer. The GGDEF domain is made of a central five-stranded β-sheet with five α-helices surrounding it. Each dimer contains an active half-site that, when combined together in a productive conformation, form the entire active site. Each half-site binds one GTP. The guanyl base forms hydrogen bonds with asparagine-173 and aspartate-182 to hold it in the active site. A magnesium2+ ion stabilizes the negative charges on the phosphate groups. When in the productive conformation, each GTP is held in close proximity with the α-phosphate groups overlapping C3 of the ribose. This conformation allows the α-phospate of one GTP to react with alcohol on C3 on the ribose of the other GTP, resulting in a cyclization of the two molecules into cyclic-dimeric-GMP.