Carboxylesterase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{STRUCTURE_4ab1| PDB=4ab1 | SIZE=350| SCENE=Journal:Acta_Cryst_F:1/Cv/2 |right|CAPTION=Human carboxylesterase 1 [[4ab1]] }} | {{STRUCTURE_4ab1| PDB=4ab1 | SIZE=350| SCENE=Journal:Acta_Cryst_F:1/Cv/2 |right|CAPTION=Human carboxylesterase 1 [[4ab1]] }} | ||
| + | <StructureSection load='4ab1' size='350' side='right' caption='Human carboxylesterase 1 (PDB code [[4ab1]]).' scene='Journal:Acta_Cryst_F:1/Cv/2'> | ||
'''Carboxylesterase''' (CE) catalyzes the conversion of a wide variety carboxylic esters to alcohol and carboxylate. The catalytic triad of CE involves serine, glutamic acid or aspartic acid and histidine. Human CE1 (hCE1) is involved in drug metabolism and activation. It catalyzes the hydrolysis of heroin and cocaine. <br /> | '''Carboxylesterase''' (CE) catalyzes the conversion of a wide variety carboxylic esters to alcohol and carboxylate. The catalytic triad of CE involves serine, glutamic acid or aspartic acid and histidine. Human CE1 (hCE1) is involved in drug metabolism and activation. It catalyzes the hydrolysis of heroin and cocaine. <br /> | ||
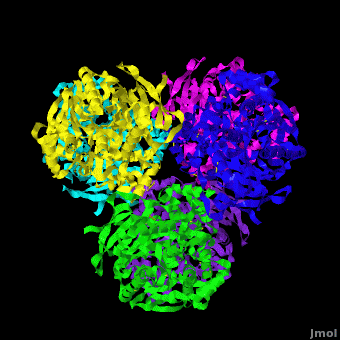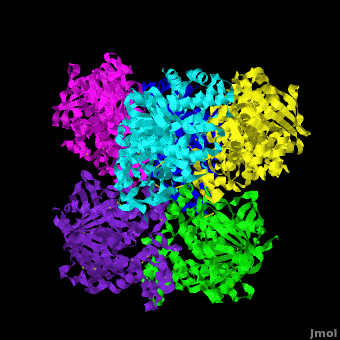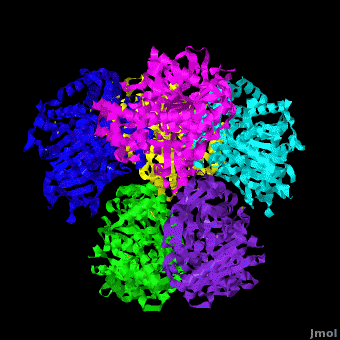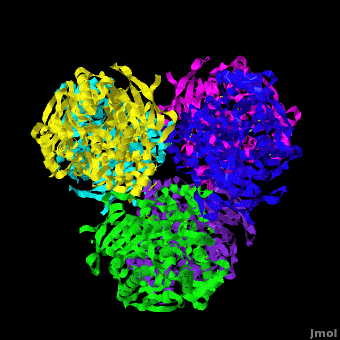
Human carboxylesterase 1 (rhCES1) has been produced in and isolated from whole ''Trichoplusia ni'' larvae. The recombinant protein was crystallized and its structure was solved to 2.2 Å resolution ([[4ab1]]). The current structure of rhCES1 represents the first published hexagonal crystal form, despite the fact that all other published examples of hCES1 structures consist of a hexamer in the asymmetric unit. <scene name='Journal:Acta_Cryst_F:1/Cv/4'>The trimer of subunits sits around one of the threefold axes</scene> found in this space group, while the three twofold axes at z = 1/4 that intersect on this axis complete the <scene name='Journal:Acta_Cryst_F:1/Cv/5'>hexamer</scene>. An <scene name='Journal:Acta_Cryst_F:1/Cv/6'>alignment of the A chain from PDB entry 2h7c with the asymmetric unit reported here</scene> gave an r.m.s. deviation of 0.42 Å for 522 Cα atoms ([[2h7c]] <font color='red'><b>is colored in red</b></font> and <span style="color:lime;background-color:black;font-weight:bold;">rhCES1 is in green</span>). An r.m.s. value of 0.47 Å (3132 Cα atoms) was obtained for the <scene name='Journal:Acta_Cryst_F:1/Cv/7'>entire 2h7c hexamer superposed with the symmetry-generated rhCES1 hexamer</scene>, indicating that the quaternary structure is essentially identical in these crystal forms isolated from cultured Sf21 cells, supporting the use of this expression system to produce recombinant enzymes for crystallization studies. | Human carboxylesterase 1 (rhCES1) has been produced in and isolated from whole ''Trichoplusia ni'' larvae. The recombinant protein was crystallized and its structure was solved to 2.2 Å resolution ([[4ab1]]). The current structure of rhCES1 represents the first published hexagonal crystal form, despite the fact that all other published examples of hCES1 structures consist of a hexamer in the asymmetric unit. <scene name='Journal:Acta_Cryst_F:1/Cv/4'>The trimer of subunits sits around one of the threefold axes</scene> found in this space group, while the three twofold axes at z = 1/4 that intersect on this axis complete the <scene name='Journal:Acta_Cryst_F:1/Cv/5'>hexamer</scene>. An <scene name='Journal:Acta_Cryst_F:1/Cv/6'>alignment of the A chain from PDB entry 2h7c with the asymmetric unit reported here</scene> gave an r.m.s. deviation of 0.42 Å for 522 Cα atoms ([[2h7c]] <font color='red'><b>is colored in red</b></font> and <span style="color:lime;background-color:black;font-weight:bold;">rhCES1 is in green</span>). An r.m.s. value of 0.47 Å (3132 Cα atoms) was obtained for the <scene name='Journal:Acta_Cryst_F:1/Cv/7'>entire 2h7c hexamer superposed with the symmetry-generated rhCES1 hexamer</scene>, indicating that the quaternary structure is essentially identical in these crystal forms isolated from cultured Sf21 cells, supporting the use of this expression system to produce recombinant enzymes for crystallization studies. | ||
| Line 6: | Line 7: | ||
For the hydrolysis of cocaine by CE see [[Cocaethylene Synthesis and Pathophysiology]]. | For the hydrolysis of cocaine by CE see [[Cocaethylene Synthesis and Pathophysiology]]. | ||
| - | + | </StructureSection> | |
==3D structures of Carboxylesterase== | ==3D structures of Carboxylesterase== | ||
Revision as of 10:32, 27 March 2017
| |||||||||||
3D structures of Carboxylesterase
Updated on 27-March-2017
References
- ↑ Greenblatt HM, Otto TC, Kirkpatrick MG, Kovaleva E, Brown S, Buchman G, Cerasoli DM, Sussman JL. Structure of recombinant human carboxylesterase 1 isolated from whole cabbage looper larvae. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Mar 1;68(Pt 3):269-72., Epub 2012 Feb 15. PMID:22442219 doi:10.1107/S1744309112003326