We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1052
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== DNA Bound State == | == DNA Bound State == | ||
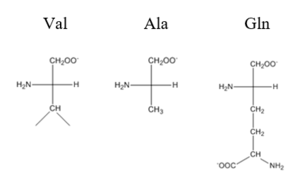
| - | The <scene name='69/694219/Serandhisresidues/2'>main DNA interactions</scene> have been found to occur at the Ser 54 and 57 along with His 58. These residues are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA. These residues are found in the N terminal of the R helix. The residues involved in the <scene name='69/694219/Dna_binding_pocket/1'>DNA binding pocket</scene> are Val 42 and Gln 53. This was experimentally determined by replacing the Gln and Val with Ala residues and measuring the binding capacity; In a previously published article <ref>DOI: 10.1073pnas.0905558106</ref>, the DNA bound state of CzrA was tested by using the known critical residues for DNA interactions. Critical residues, Gln53, Val42, Ser54, Ser57, and His58, were replaced with Ala and then compared to the kinetics of the wild type protein. Replacing only the Q53 and V42 residues resulted in an 11-fold and 160-fold decrease in K<sub>a</sub>, respectively. Other residues such as S54, S57, and H58 were also replaced with Ala residues, and it was found that these mutations caused binding similar to the fully inhibited Zn<sup>2+</sup> bound state. Table 1 in this article | + | The <scene name='69/694219/Serandhisresidues/2'>main DNA interactions</scene> have been found to occur at the Ser 54 and 57 along with His 58. These residues are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA. These residues are found in the N terminal of the R helix. The residues involved in the <scene name='69/694219/Dna_binding_pocket/1'>DNA binding pocket</scene> are Val 42 and Gln 53. This was experimentally determined by replacing the Gln and Val with Ala residues and measuring the binding capacity; In a previously published article <ref>DOI: 10.1073pnas.0905558106</ref>, the DNA bound state of CzrA was tested by using the known critical residues for DNA interactions. Critical residues, Gln53, Val42, Ser54, Ser57, and His58, were replaced with Ala and then compared to the kinetics of the wild type protein. Replacing only the Q53 and V42 residues resulted in an 11-fold and 160-fold decrease in K<sub>a</sub>, respectively. Other residues such as S54, S57, and H58 were also replaced with Ala residues, and it was found that these mutations caused binding similar to the fully inhibited Zn<sup>2+</sup> bound state. Table 1 in this same article shows the different K<sub>observed</sub>, and the measured decrease in K<sub>observed</sub> for each mutation. The bind between the DNA and the protein can be attributed to losing certain intermolecular forces such as possible hydrogen bonding when changing from Gln and Ala, and a loss of London Dispersion forces in the Val to Ala change. [[Image:Capture01.PNG|300px|center|thumb| Comparison of Val, Ala, and Gln residues]] |
The differences in binding favorability can also be seen when comparing the ΔG for the Apo-state vs. the DNA bound state and the Zinc vs. the Zinc and DNA bound state. These ΔGs were found to be -15.2kcal/mol and -9kcal/mol respectively<ref>DOI: 10.1021/ja208047b</ref>. This agrees with previously published data showing the Zinc binding inhibits the affinity the protein has to DNA. | The differences in binding favorability can also be seen when comparing the ΔG for the Apo-state vs. the DNA bound state and the Zinc vs. the Zinc and DNA bound state. These ΔGs were found to be -15.2kcal/mol and -9kcal/mol respectively<ref>DOI: 10.1021/ja208047b</ref>. This agrees with previously published data showing the Zinc binding inhibits the affinity the protein has to DNA. | ||
Revision as of 12:51, 28 March 2017
|
Contents |
CzrA
Biological Function
Structural Overview
DNA Bound State
The have been found to occur at the Ser 54 and 57 along with His 58. These residues are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA. These residues are found in the N terminal of the R helix. The residues involved in the are Val 42 and Gln 53. This was experimentally determined by replacing the Gln and Val with Ala residues and measuring the binding capacity; In a previously published article [1], the DNA bound state of CzrA was tested by using the known critical residues for DNA interactions. Critical residues, Gln53, Val42, Ser54, Ser57, and His58, were replaced with Ala and then compared to the kinetics of the wild type protein. Replacing only the Q53 and V42 residues resulted in an 11-fold and 160-fold decrease in Ka, respectively. Other residues such as S54, S57, and H58 were also replaced with Ala residues, and it was found that these mutations caused binding similar to the fully inhibited Zn2+ bound state. Table 1 in this same article shows the different Kobserved, and the measured decrease in Kobserved for each mutation. The bind between the DNA and the protein can be attributed to losing certain intermolecular forces such as possible hydrogen bonding when changing from Gln and Ala, and a loss of London Dispersion forces in the Val to Ala change.The differences in binding favorability can also be seen when comparing the ΔG for the Apo-state vs. the DNA bound state and the Zinc vs. the Zinc and DNA bound state. These ΔGs were found to be -15.2kcal/mol and -9kcal/mol respectively[2]. This agrees with previously published data showing the Zinc binding inhibits the affinity the protein has to DNA.
Zinc Ligand(s)
</StructureSection>
References
1. Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 18177-18182
2. Chakravorty D., Giedroc D., Merz K., Lee C., Wang B. (2012). Simulations of Allosteric Motions in the Zinc Sensor CzrA. JCAS 134 3367-3376