Sandbox Reserved 1069
From Proteopedia
| Line 9: | Line 9: | ||
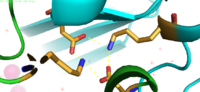
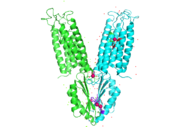
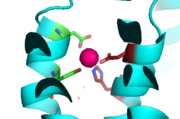
Yiip's complete protein structure is identified as a dimer. Each [https://en.wikipedia.org/wiki/Monomer monomer] structure is classified into two different domains, the Trans-Membrane Domain (TMD) and C-Terminus Domain (CTD). The TMD consists of six helices, forming binding site A, four of which are oriented in a parallel manner, with respect to each other, while the remaining two are aligned anti parallel to this four helix cluster. A "<scene name='69/694234/Bridge/1'>salt bridge</scene>" or "charge interlock" consisting of four amino acid residues, two [https://en.wikipedia.org/wiki/Lysine Lysines] (Lys 77) and two [https://en.wikipedia.org/wiki/Aspartic_acid Aspartates] (Asp 207), forms a junction that both monomers of Yiip converge at, forming a pivot point for conformation changes. A large portion of the protein containing binding site C, approximately 30 Å in length<sup>[1]</sup>, protrudes into the cytoplasm functioning as a zinc sensor within the cell. | Yiip's complete protein structure is identified as a dimer. Each [https://en.wikipedia.org/wiki/Monomer monomer] structure is classified into two different domains, the Trans-Membrane Domain (TMD) and C-Terminus Domain (CTD). The TMD consists of six helices, forming binding site A, four of which are oriented in a parallel manner, with respect to each other, while the remaining two are aligned anti parallel to this four helix cluster. A "<scene name='69/694234/Bridge/1'>salt bridge</scene>" or "charge interlock" consisting of four amino acid residues, two [https://en.wikipedia.org/wiki/Lysine Lysines] (Lys 77) and two [https://en.wikipedia.org/wiki/Aspartic_acid Aspartates] (Asp 207), forms a junction that both monomers of Yiip converge at, forming a pivot point for conformation changes. A large portion of the protein containing binding site C, approximately 30 Å in length<sup>[1]</sup>, protrudes into the cytoplasm functioning as a zinc sensor within the cell. | ||
| - | YiiP is a homodimer with each protomer consisting of 238 residues. YiiP has a "Y" shape <scene name='75/756372/Newmainpic/1'> conformation</scene> with two different functional conformations. A total of six helices comprise the transmembrane portion of each <scene name='75/756372/Sixhelices/1'>protomer</scene>. Four of these helices are bundled together while the remaining two are oriented antiparallel to the <scene name='75/756372/2antiparallel/1'>bundle</scene>. Movement of these helices play a role in the function of YiiP. An interlocked salt bridge connects the two domains with the Lys77 and the Asp207 from each protomer - visualized <scene name='75/756372/Saltbridgeclose/1'>here</scene>.This salt bridge acts as the hinge for the conformational changes that YiiP undergoes. YiiP has three zinc binding sites, two of which are known to play an active role in the function of YiiP. Site A (link) sits in extracellular space outside of the cell, while site C is situated inside of the cell to act as a sensor of intracellular zinc concentrations. | + | YiiP is a homodimer with each protomer consisting of 238 residues. YiiP has a "Y" shape <scene name='75/756372/Newmainpic/1'> conformation</scene> with two different functional conformations. A total of six helices comprise the transmembrane portion of each <scene name='75/756372/Sixhelices/1'>protomer</scene>. Four of these helices are bundled together while the remaining two are oriented antiparallel to the <scene name='75/756372/2antiparallel/1'>bundle</scene>. Movement of these helices play a role in the function of YiiP. An interlocked salt bridge connects the two domains with the Lys77 and the Asp207 from each protomer - visualized <scene name='75/756372/Saltbridgeclose/1'>here</scene>.This [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridge] acts as the hinge for the conformational changes that YiiP undergoes. YiiP has three zinc binding sites, two of which are known to play an active role in the function of YiiP. Site A (link) sits in extracellular space outside of the cell, while site C is situated inside of the cell to act as a sensor of intracellular zinc concentrations. |
=== Structural highlights === | === Structural highlights === | ||
Revision as of 21:03, 29 March 2017
Introduction
Zinc transporter YiiP is an integral membrane protein found in the membrane of Esherichia coli. YiiP is a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological realm. These diffusion facilitators export divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which is biologically relevant because while these metals are necessary for different biological functions, they can prove fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response. Zinc transporters help keep homeostatic balance of zinc in cells and excessive zinc concentrations have been noted in cases of high beta-amyloid deposition contributing to Alzheimer's disease (CITE: http://science.sciencemag.org/content/317/5845/1746.full While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researcher's better understand the cation diffusion facilitator equivalents in eukaryotic cells.
Structure
| |||||||||||