Sandbox Reserved 1069
From Proteopedia
| Line 30: | Line 30: | ||
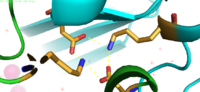
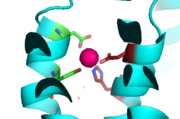
<scene name='69/694236/Site_c/1'>Binding Site C</scene> has vastly opposite properties from what is seen in binding site A. It is located on the TM2-TM3 loop on the cytoplasmic membrane and between the two C-terminus domain interfaces. Here, there is a binuclear coordination of zinc between the <scene name='69/694236/Asp285/3'>Asp285</scene> residue that bridges the zinc ions together and the four coordinating residues (His232, His248, His283 and His261). The Asp285 residue is conserved, meaning it does not have outer shell constraints. However, the four histidine residues all have outer shell constraints. These constraints consist of hydrogen bonds to the residues surrounding the binding site. These hydrogen bonds can form bidentate bonds, which means that the hydrogen bond attaches to a metal in two places. These bonds in turn create an extensive network of interactions at the CTD interface, and it is these interactions that allow for stability and strengthening of the CTD-CTD association. | <scene name='69/694236/Site_c/1'>Binding Site C</scene> has vastly opposite properties from what is seen in binding site A. It is located on the TM2-TM3 loop on the cytoplasmic membrane and between the two C-terminus domain interfaces. Here, there is a binuclear coordination of zinc between the <scene name='69/694236/Asp285/3'>Asp285</scene> residue that bridges the zinc ions together and the four coordinating residues (His232, His248, His283 and His261). The Asp285 residue is conserved, meaning it does not have outer shell constraints. However, the four histidine residues all have outer shell constraints. These constraints consist of hydrogen bonds to the residues surrounding the binding site. These hydrogen bonds can form bidentate bonds, which means that the hydrogen bond attaches to a metal in two places. These bonds in turn create an extensive network of interactions at the CTD interface, and it is these interactions that allow for stability and strengthening of the CTD-CTD association. | ||
| + | |||
| + | ==Mechanism of Transport== | ||
| + | |||
| + | YiiP's ability to export Zn<sup>2+</sup> from the cytoplasm is best described as an alternating access mechanism with Zn<sup>2+</sup>/H<sup>+</sup> antiport. YiiP has 2 major structural conformations as shown by the crystallized structures [[3H90]] and [[3J1Z]] (a YiiP homolog derived from ''Shewanella oneidensis''). 3H90 shows YiiP in its outward-facing conformation and 3J1Z shows the YiiP homolog in an inward-facing conformation. | ||
| + | When YiiP is saturated with Zn<sup>2+</sup> it seems to favor the <scene name='69/694233/Outward-facing_conformation/2'>outward-facing conformation</scene> whereas when active sites are either empty or bound to H<sup>+</sup> the <scene name='69/694233/Outward-facing_conformation/1'>inward-facing conformation</scene> is favored. This drives the export of Zn<sup>2+</sup> from the cytoplasm and enhances the coupling of the proton-motive force. Although YiiP exists as a homodimer both monomers can undergo conformation change independent of one other to | ||
| + | produce the alternating access mechanism. | ||
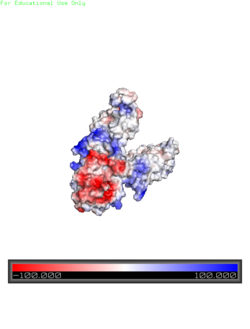
== Electrostatic Interactions == | == Electrostatic Interactions == | ||
| Line 39: | Line 45: | ||
In contrast the main purpose of conformation change in the CTD is to stabilize the YiiP dimer and acts as a Zn<sup>2+</sup> sensor. This is possible because of the flexible loop that links the TMD and the CTD. This loop harbors the charge interlock which serves as a hinge that allows movement of the CTD. Using FRET to measure the distance between the CTD of each monomer fluorescence quenching was observed as the concentration Zn<sup>2+</sup> increased, which supports that idea that Zn<sup>2+</sup> induces a stabilizing conformation change in the CTD. | In contrast the main purpose of conformation change in the CTD is to stabilize the YiiP dimer and acts as a Zn<sup>2+</sup> sensor. This is possible because of the flexible loop that links the TMD and the CTD. This loop harbors the charge interlock which serves as a hinge that allows movement of the CTD. Using FRET to measure the distance between the CTD of each monomer fluorescence quenching was observed as the concentration Zn<sup>2+</sup> increased, which supports that idea that Zn<sup>2+</sup> induces a stabilizing conformation change in the CTD. | ||
| - | ==Mechanism of Transport== | ||
| - | YiiP's ability to export Zn<sup>2+</sup> from the cytoplasm is best described as an alternating access mechanism with Zn<sup>2+</sup>/H<sup>+</sup> antiport. YiiP has 2 major structural conformations as shown by the crystallized structures [[3H90]] and [[3J1Z]] (a YiiP homolog derived from ''Shewanella oneidensis''). 3H90 shows YiiP in its outward-facing conformation and 3J1Z shows the YiiP homolog in an inward-facing conformation. | ||
| - | When YiiP is saturated with Zn<sup>2+</sup> it seems to favor the <scene name='69/694233/Outward-facing_conformation/2'>outward-facing conformation</scene> whereas when active sites are either empty or bound to H<sup>+</sup> the <scene name='69/694233/Outward-facing_conformation/1'>inward-facing conformation</scene> is favored. This drives the export of Zn<sup>2+</sup> from the cytoplasm and enhances the coupling of the proton-motive force. Although YiiP exists as a homodimer both monomers can undergo conformation change independent of one other to | ||
| - | produce the alternating access mechanism. | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:33, 29 March 2017
Introduction
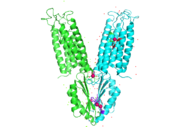
Zinc transporter is an integral membrane protein found in the membrane of Esherichia coli and a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological real, their primary function being the export of divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which is biologically relevant because while these metals are necessary for different biological functions, they can prove fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response. While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researcher's better understand the cation diffusion facilitator equivalents in eukaryotic cells.
| |||||||||||