Sandbox Reserved 1069
From Proteopedia
| Line 8: | Line 8: | ||
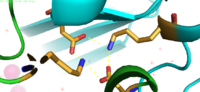
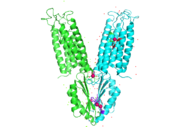
YiiP is a homodimer, with each monomer consisting of 238 residues [https://en.wikipedia.org/wiki/Protein_dimer (protein dimer)] with a TransMembrane (<scene name='69/694236/Tmd/1'>TMD</scene>) and C-Terminal (<scene name='69/694236/Ctd/1'>CTD</scene>) domain that are connected via a charge interlocking mechanism located on a flexible loop. The TMD, where Zn<sup>2+</sup> binding site A resides, consists of a total of six helices in each <scene name='75/756372/Sixhelices/1'>monomer</scene>. Four of these helices are bundled together while the remaining two are oriented antiparallel to the <scene name='75/756372/2antiparallel/1'>bundle</scene>. Movement of these helices play a role in the function of YiiP. A large portion of the protein containing binding site C, the CTD, approximately 30 Å in length<sup>[https://www.bnl.gov/isd/documents/71335.pdf]</sup>, protrudes into the cytoplasm functioning as a zinc sensor within the cell. | YiiP is a homodimer, with each monomer consisting of 238 residues [https://en.wikipedia.org/wiki/Protein_dimer (protein dimer)] with a TransMembrane (<scene name='69/694236/Tmd/1'>TMD</scene>) and C-Terminal (<scene name='69/694236/Ctd/1'>CTD</scene>) domain that are connected via a charge interlocking mechanism located on a flexible loop. The TMD, where Zn<sup>2+</sup> binding site A resides, consists of a total of six helices in each <scene name='75/756372/Sixhelices/1'>monomer</scene>. Four of these helices are bundled together while the remaining two are oriented antiparallel to the <scene name='75/756372/2antiparallel/1'>bundle</scene>. Movement of these helices play a role in the function of YiiP. A large portion of the protein containing binding site C, the CTD, approximately 30 Å in length<sup>[https://www.bnl.gov/isd/documents/71335.pdf]</sup>, protrudes into the cytoplasm functioning as a zinc sensor within the cell. | ||
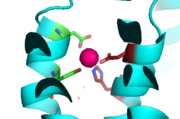
| - | Site A is located in both TMDs of the protein, while site C is located in the CTD, and site B is located at the junction of the domains join. Both TMD are composed of 6 helices, 4 of which ( | + | Site A is located in both TMDs of the protein, while site C is located in the CTD, and site B is located at the junction of the domains join. Both TMD are composed of 6 helices, 4 of which (TM1, TM2, TM4, TM5) pivot about the ion binding site A. Zn<sup>2+</sup> binding at site C helps hold the CTD together and is thought to stabilize conformational changes in YiiP. YiiP has two different functional conformations which dictates whether or not YiiP is open to the periplasm or the cytoplasm. An interlocked salt bridge connects the two domains with the Lys77 and the Asp207 from each monomer. This [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridge] acts as the hinge for the conformational changes that YiiP undergoes. |
| Line 38: | Line 38: | ||
===Zn<sup>2+</sup> Induced Conformation Change=== | ===Zn<sup>2+</sup> Induced Conformation Change=== | ||
| - | Conformation changes occur in the TMD and CTD, both of which are heavily influenced by the presence of Zn<sup>2+</sup>. The conformation change directly involved with Zn<sup>2+</sup>/H<sup>+</sup> antiport occurs in the TMD as helix pivoting controls what environment site A is available to. Conformation change occurs when the transmembrane helix pairs | + | Conformation changes occur in the TMD and CTD, both of which are heavily influenced by the presence of Zn<sup>2+</sup>. The conformation change directly involved with Zn<sup>2+</sup>/H<sup>+</sup> antiport occurs in the TMD as helix pivoting controls what environment site A is available to. Conformation change occurs when the transmembrane helix pairs TM1, TM2, TM4, and TM5 pivot around cation binding site. It is believed that the energy for TMD conformation change comes from energy of binding each substrate. Changing to the outward from the inward-facing conformation causes a shift in <scene name='69/694233/Transmembrane_helix_5/2'>TM5</scene> which disrupts the tetrahedral geometry of active site A. This in turn decreases binding affinity site A has for Zn<sup>2+</sup> making export to the periplasm possible. After Zn<sup>2+</sup> is exported and site A is either empty or bound to hydrogen change back to the inward-facing conformation is favored. |
In contrast the main purpose of conformation change in the CTD is to stabilize the YiiP dimer and acts as a Zn<sup>2+</sup> sensor. This is possible because of the flexible loop that links the TMD and the CTD. This loop harbors the charge interlock which serves as a hinge that allows movement of the CTD. Using [https://en.wikipedia.org/wiki/F%C3%B6rster_resonance_energy_transfer FRET] to measure the distance between the CTD of each monomer fluorescence quenching was observed as the concentration Zn<sup>2+</sup> increased, which supports that idea that Zn<sup>2+</sup> induces a stabilizing conformation change in the CTD. | In contrast the main purpose of conformation change in the CTD is to stabilize the YiiP dimer and acts as a Zn<sup>2+</sup> sensor. This is possible because of the flexible loop that links the TMD and the CTD. This loop harbors the charge interlock which serves as a hinge that allows movement of the CTD. Using [https://en.wikipedia.org/wiki/F%C3%B6rster_resonance_energy_transfer FRET] to measure the distance between the CTD of each monomer fluorescence quenching was observed as the concentration Zn<sup>2+</sup> increased, which supports that idea that Zn<sup>2+</sup> induces a stabilizing conformation change in the CTD. | ||
Revision as of 22:05, 29 March 2017
Introduction
Zinc transporter is an integral membrane protein found in the membrane of Esherichia coli and a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological real, their primary function being the export of divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which is biologically relevant because while these metals are necessary for different biological functions, they can prove fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response. While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researcher's better understand the cation diffusion facilitator equivalents in eukaryotic cells.
| |||||||||||